Hemodynamic adaptation of heart failure to percutaneous venoarterial extracorporeal circulatory supports
- PMID: 32901493
- PMCID: PMC8549913
- DOI: 10.33549/physiolres.934332
Hemodynamic adaptation of heart failure to percutaneous venoarterial extracorporeal circulatory supports
Abstract
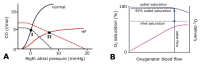
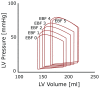
Extracorporeal life support (ECLS) is a treatment modality that provides prolonged blood circulation, gas exchange and can partially support or fully substitute functions of heart and lungs in patients with severe but potentially reversible cardiopulmonary failure refractory to conventional therapy. Due to high-volume bypass, the extracorporeal flow is interacting with native cardiac output. The pathophysiology of circulation and ECLS support reveals significant effects on arterial pressure waveforms, cardiac hemodynamics, and myocardial perfusion. Moreover, it is still subject of research, whether increasing stroke work caused by the extracorporeal flow is accompanied by adequate myocardial oxygen supply. The left ventricular (LV) pressure-volume mechanics are reflecting perfusion and loading conditions and these changes are dependent on the degree of the extracorporeal blood flow. By increasing the afterload, artificial circulation puts higher demands on heart work with increasing myocardial oxygen consumption. Further, this can lead to LV distention, pulmonary edema, and progression of heart failure. Multiple methods of LV decompression (atrial septostomy, active venting, intra-aortic balloon pump, pulsatility of flow) have been suggested to relieve LV overload but the main risk factors still remain unclear. In this context, it has been recommended to keep the rate of circulatory support as low as possible. Also, utilization of detailed hemodynamic monitoring has been suggested in order to avoid possible harm from excessive extracorporeal flow.
Conflict of interest statement
There is no conflict of interest.
Figures
Similar articles
-
Ten years of our translational research in the field of veno-arterial extracorporeal membrane oxygenation.Physiol Res. 2022 Dec 31;71(S2):S163-S178. doi: 10.33549/physiolres.934999. Physiol Res. 2022. PMID: 36647905 Free PMC article. Review.
-
Extracorporeal membrane oxygenation versus counterpulsatile, pulsatile, and continuous left ventricular unloading for pediatric mechanical circulatory support.Pediatr Crit Care Med. 2013 Nov;14(9):e424-37. doi: 10.1097/PCC.0b013e3182a551b0. Pediatr Crit Care Med. 2013. PMID: 24108116 Free PMC article.
-
Comparison of Circulatory Unloading Techniques for Venoarterial Extracorporeal Membrane Oxygenation.ASAIO J. 2021 Jun 1;67(6):623-631. doi: 10.1097/MAT.0000000000001268. ASAIO J. 2021. PMID: 33074863
-
Increasing venoarterial extracorporeal membrane oxygenation flow puts higher demands on left ventricular work in a porcine model of chronic heart failure.J Transl Med. 2020 Feb 13;18(1):75. doi: 10.1186/s12967-020-02250-x. J Transl Med. 2020. PMID: 32054495 Free PMC article.
-
Left Ventricular Unloading During Extracorporeal Life Support: Current Practice.J Card Fail. 2022 Aug;28(8):1326-1336. doi: 10.1016/j.cardfail.2021.12.002. Epub 2021 Dec 20. J Card Fail. 2022. PMID: 34936896 Review.
Cited by
-
Clinical Efficacy of Blood Ultrafiltration Therapy in Patients with Acute Decompensated Chronic Heart Failure Running Title: Blood Ultrafiltration Therapy for Heart Failure.Physiol Res. 2023 Dec 31;72(6):701-706. doi: 10.33549/physiolres.935073. Physiol Res. 2023. PMID: 38215058 Free PMC article.
-
Ten years of our translational research in the field of veno-arterial extracorporeal membrane oxygenation.Physiol Res. 2022 Dec 31;71(S2):S163-S178. doi: 10.33549/physiolres.934999. Physiol Res. 2022. PMID: 36647905 Free PMC article. Review.
References
-
- AISSAOUI N, GUEROT E, COMBES A, DELOUCHE A, CHASTRE J, LEPRINCE P, LEGER P, DIEHL JL, FAGON JY, DIEBOLD B. Two-dimensional strain rate and Doppler tissue myocardial velocities: analysis by echocardiography of hemodynamic and functional changes of the failed left ventricle during different degrees of extracorporeal life support. J Am Soc Echocardiogr. 2012;25:632–640. doi: 10.1016/j.echo.2012.02.009. - DOI - PubMed
