Testosterone antagonizes paraquat-induced cardiomyocyte senescence via the mIGF-1/SIRT1 signaling pathway
- PMID: 32901689
- PMCID: PMC7485312
- DOI: 10.1590/1414-431X20209849
Testosterone antagonizes paraquat-induced cardiomyocyte senescence via the mIGF-1/SIRT1 signaling pathway
Abstract
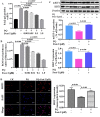
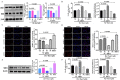
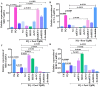
Testosterone has been demonstrated to antagonize doxorubicin-induced cardiomyocyte senescence. However, whether testosterone prevents the paraquat-induced cardiomyocyte senescence is largely unknown. The detection of SA-β-gal activity was performed using senescence β-gal staining kit and the reactive oxygen species levels were determined by reactive oxygen species assay kit. The plasmids for insulin-like growth factor 1 shRNA (sh-mIGF-1), sirtuin-1 shRNA (sh-SIRT1), scramble shRNA (sh-NC), overexpressing mIGF-1 (mIGF-1), overexpressing SIRT1 (SIRT1), and negative controls (NC) were obtained for this study. The expression of target genes was detected using quantitative real-time PCR, immunolabeling, and western blot. We found that testosterone significantly delayed the paraquat-induced HL-1 cardiomyocyte senescence as evidenced by decreasing senescence-associated β-galactosidase activity and reactive oxygen species generation, which were accompanied by the up-regulated expression of mIGF-1 and SIRT1. RNA interference to reduce mIGF-1 and SIRT1 expression showed that testosterone prevented paraquat-induced HL-1 senescence via the mIGF-1/SIRT1 signaling pathway. Furthermore, myocardial contraction was evaluated by expression of genes of the contractile proteins/enzymes, such as α-myosin heavy chain 6 (MHC6), α-myosin heavy chain 7 (MHC7), α-skeletal actin (ACTA-1), and sarco/endoplasmic reticulum calcium ATPase-2 (SERCA2). Testosterone adjusted the above four gene expressions and the adjustment was blocked by mIGF-1 or SIRT1 inhibition. Our findings suggested that the mIGF-1/SIRT1 signaling pathway mediated the protective function of testosterone against the HL-1 cardiomyocyte senescence by paraquat, which provided new clues for the mechanisms underlying the anti-aging role of testosterone in cardiomyocytes.
Figures
Similar articles
-
Local IGF-1 isoform protects cardiomyocytes from hypertrophic and oxidative stresses via SirT1 activity.Aging (Albany NY). 2009 Dec 10;2(1):43-62. doi: 10.18632/aging.100107. Aging (Albany NY). 2009. PMID: 20228935 Free PMC article.
-
mIGF-1/JNK1/SirT1 signaling confers protection against oxidative stress in the heart.Aging Cell. 2012 Feb;11(1):139-49. doi: 10.1111/j.1474-9726.2011.00766.x. Epub 2011 Dec 11. Aging Cell. 2012. PMID: 22051242
-
The TRPA1 channel is a cardiac target of mIGF-1/SIRT1 signaling.Am J Physiol Heart Circ Physiol. 2014 Oct 1;307(7):H939-44. doi: 10.1152/ajpheart.00150.2014. Epub 2014 Aug 8. Am J Physiol Heart Circ Physiol. 2014. PMID: 25108014
-
Cardioprotective mIGF-1/SIRT1 signaling induces hypertension, leukocytosis and fear response in mice.Aging (Albany NY). 2012 Jun;4(6):402-16. doi: 10.18632/aging.100464. Aging (Albany NY). 2012. PMID: 22691943 Free PMC article.
-
Testosterone protects cardiomyocytes against hydrogen peroxide-induced aging by upregulating IGF1 and SIRT1 pathways.Physiol Int. 2022 Aug 24. doi: 10.1556/2060.2022.00191. Online ahead of print. Physiol Int. 2022. PMID: 36001411
Cited by
-
Secreted Cytokines within the Urine of AKI Patients Modulate TP53 and SIRT1 Levels in a Human Podocyte Cell Model.Int J Mol Sci. 2023 May 4;24(9):8228. doi: 10.3390/ijms24098228. Int J Mol Sci. 2023. PMID: 37175937 Free PMC article.
References
-
- Araujo AB, O'Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts male aging study. J Clin Endocrinol Metab. 2004;89:5920–5926. doi: 10.1210/jc.2003-031719. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

