Fear conditioning prompts sparser representations of conditioned threat in primary visual cortex
- PMID: 32901822
- PMCID: PMC7647380
- DOI: 10.1093/scan/nsaa122
Fear conditioning prompts sparser representations of conditioned threat in primary visual cortex
Abstract
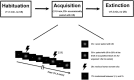
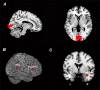
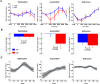
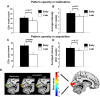
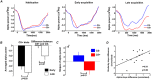
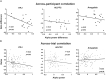
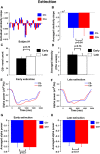
Repeated exposure to threatening stimuli alters sensory responses. We investigated the underlying neural mechanism by re-analyzing previously published simultaneous electroencephalogram-functional magnetic resonance imaging (EEG-fMRI) data from humans viewing oriented gratings during Pavlovian fear conditioning. In acquisition, one grating (CS+) was paired with a noxious noise, the unconditioned stimulus (US). The other grating (CS-) was never paired with the US. In habituation, which preceded acquisition, and in extinction, the same two gratings were presented without US. Using fMRI multivoxel patterns in primary visual cortex during habituation as reference, we found that during acquisition, aversive learning selectively prompted systematic changes in multivoxel patterns evoked by CS+. Specifically, CS+ evoked voxel patterns in V1 became sparser as aversive learning progressed, and the sparsified pattern appeared to be preserved in extinction. Concomitant with the voxel pattern changes, occipital alpha oscillations were increasingly more desynchronized during CS+ (but not CS-) trials. Across acquisition trials, the rate of change in CS+-related alpha desynchronization was correlated with the rate of change in multivoxel pattern representations of CS+. Furthermore, alpha oscillations co-varied with blood-oxygen-level-dependent (BOLD) data in the ventral attention network, but not with BOLD in the amygdala. Thus, fear conditioning prompts persistent sparsification of voxel patterns evoked by threat, likely mediated by attention-related mechanisms.
Keywords: alpha oscillations; attention; fear conditioning; sparsification; visual representation.
© The Author(s) 2020. Published by Oxford University Press.
Figures

References
-
- Allen P.J., Polizzi G., Krakow K., Fish D.R., Lemieux L. (1998). Identification of EEG events in the MR scanner: the problem of pulse artifact and a method for its subtraction. NeuroImage, 8(3), 229–39. Headley and Weinberger, 2011; Moran and Katz, 2014. - PubMed
-
- Amaral D.G., Behniea H., Kelly J.L. (2003). Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience, 118(4), 1099–120. - PubMed
-
- Armony J.L., Dolan R.J. (2002). Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia, 40(7), 817–26. - PubMed

