Heterogeneous nuclear ribonucleoprotein K is overexpressed and contributes to radioresistance irrespective of HPV status in head and neck squamous cell carcinoma
- PMID: 32901844
- PMCID: PMC7521550
- DOI: 10.3892/ijmm.2020.4718
Heterogeneous nuclear ribonucleoprotein K is overexpressed and contributes to radioresistance irrespective of HPV status in head and neck squamous cell carcinoma
Abstract
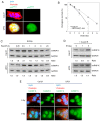
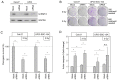
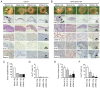
Radiotherapy is a major treatment option for head and neck squamous cell carcinoma (HNSCC). However, the success of radiotherapy is limited by tumor cell resistance to ionizing radiation (IR). Clinical studies have demonstrated an overall improved prognosis and higher susceptibility to radiotherapy of high‑risk human papillomavirus (HPV)‑associated HNSCC compared with classic HNSCC, as well as worse overall survival for male HNSCC patients. Overexpression of heterogeneous nuclear ribonucleoprotein (hnRNP) K has been associated with resistance to radiotherapy in melanoma and colorectal carcinoma. The aim of the present study was to analyze the impact of hnRNP K expression on the aggressiveness and radioresistance of HNSCC with respect to patient sex and HPV status. Immunohistochemical staining of HNSCC tissue specimens revealed elevated hnRNP K levels compared with those in the non‑neoplastic epithelium. Cytoplasmic hnRNP K accumulation was associated with advanced tumor stage and male sex. Exposure of HNSCC cells to IR was followed by rapid upregulation of hnRNP K at the protein level, along with re‑localization from the tumor cell nucleus to the cytoplasm. siRNA‑based knockdown of hnRNP K induced apoptosis and abolished tumor formation after xenotransplantation of HNSCC cells onto the chick egg chorioallantoic membrane (CAM). The observed effects were independent of the respective HPV status of the cell lines. These results indicated a tumorigenic and anti‑apoptotic role of hnRNP K in HNSCC, which appeared to be enhanced in male patients and contributed to the radioresistance of these tumors. However, the radioprotective effects of hnRNP K were found to be independent of the tumor's HPV status.
Figures

References
-
- Franceschi S, Talamini R, Barra S, Baron AE, Negri E, Bidoli E, Serraino D, La Vecchia C. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res. 1990;50:6502–6507. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

