Autophagy inhibition enhances the inhibitory effects of ursolic acid on lung cancer cells
- PMID: 32901853
- PMCID: PMC7521584
- DOI: 10.3892/ijmm.2020.4714
Autophagy inhibition enhances the inhibitory effects of ursolic acid on lung cancer cells
Abstract
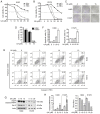
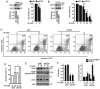
The aim of the present study was to identify natural compounds that bear significant anti‑tumor activity. Thus, the effects of 63 small molecules that were isolated from traditional Chinese medicinal herbs on A549 human non‑small cell lung cancer (NSCLC) and MCF‑7 breast cancer cells were examined. It was found that ursolic acid (UA), a natural pentacyclic triterpenoid, exerted significant inhibitory effect on these cells. Further experiments revealed that UA inhibited the proliferation of various lung cancer cells, including the NSCLC cells, H460, H1975, A549, H1299 and H520, the human small cell lung cancer (SCLC) cells, H82 and H446, and murine Lewis lung carcinoma (LLC) cells. UA induced the apoptosis and autophagy of NSCLC cells. The inhibition of the mammalian target of rapamycin (mTOR) signaling pathway, but not the activation of the extracellular signal‑regulated kinase 1/2 (ERK1/2) signaling pathway contributed to the UA‑induced autophagy of NSCLC cells. Moreover, the inhibition of autophagy by chloroquine (CQ) or siRNA for autophagy‑related gene 5 (ATG5) enhanced the UA‑induced inhibition of cell proliferation and promotion of apoptosis, indicating that UA‑induced autophagy is a pro‑survival mechanism in NSCLC cells. On the whole, these findings suggest that combination treatment with autophagy inhibitors may be a novel strategy with which enhance the antitumor activity of UA in lung cancer.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

