Distinct microglial and macrophage distribution patterns in the concentric and lamellar lesions in Baló's disease and neuromyelitis optica spectrum disorders
- PMID: 32902014
- PMCID: PMC8018076
- DOI: 10.1111/bpa.12898
Distinct microglial and macrophage distribution patterns in the concentric and lamellar lesions in Baló's disease and neuromyelitis optica spectrum disorders
Abstract
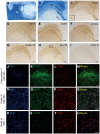
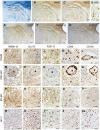
TMEM119 and purinergic receptor P2Y12 (P2RY12), which are not expressed by recruited peripheral blood macrophages, are proposed to discriminate microglia from macrophages. Therefore, we investigated the distribution patterns of microglia and macrophages in 10 concentric lesions from four autopsied Baló's disease cases and one neuromyelitis optica spectrum disorder (NMOSD) case, using quantitative immunohistochemistry for the markers TMEM119, P2RY12, CD68, CD163 and GLUT5. Three cases with Baló's disease had distal oligodendrogliopathy (DO) showing preferential loss of myelin-associated glycoprotein and early active demyelination in the outermost demyelinating layer (termed DMY-MO). In DMY-MO with DO, TMEM119-positive activated microglia expressing upregulated GLUT5 but markedly downregulated P2RY12 were significantly increased. These activated microglia expressed inducible nitric oxide synthase. Oligodendrocytes and their precursors showed apoptotic-like nuclear condensation in DMY-MO. TMEM119-negative and CD68/CD163-positive macrophages were distributed throughout the lesion center of DMY-MO with DO and these cells demonstrated foamy morphology only in the inner portion but not in the outer portion. In concentric demyelinating lesions from another Baló's case and lamellar demyelinating lesions in an NMOSD case, which had late active demyelination without DO, the densities of TMEM119-, GLUT5- and P2RY12-positive microglia with ramified morphology were significantly increased in myelinated layers but not in demyelinating layers. In particular, in the NMOSD case, TMEM119-positive microglia were confined to the outer portion of the myelinated layers. CD68-positive macrophages with foamy morphology also expressing CD163 accumulated in myelinated as well as in demyelinated layers. These findings suggest that activated microglia expressing TMEM119 and GLUT5, but not P2RY12, are associated with apoptosis of oligodendrocytes in the leading edge of Baló's concentric lesions with DO, whereas TMEM119-, GLUT5- and P2RY12-positive microglia with ramified morphology are associated with myelin preservation in concentric lesions without DO in Baló's disease and NMOSD. These two types of microglia appear to play distinct roles in the formation of concentric lesions.
Keywords: Baló's disease; distal oligodendrogliopathy; macrophage; microglia; neuromyelitis optica spectrum disorders.
© 2020 International Society of Neuropathology.
Conflict of interest statement
Dr. Kira received consultant fees, speaking fees and/or honoraria from Novartis Pharma, Mitsubishi Tanabe Pharma, Boehringer Ingelheim, Teijin Pharma, Takeda Pharmaceutical Company, Otsuka Pharmaceutical, Astellas Pharma, Pfizer Japan, Sumitomo Dainippon Pharma and Eisai. Other authors declare that they have no conflict of interest.
Figures



Similar articles
-
[Recent progress in multiple sclerosis research: astrocytopathy in demyelinating diseases].Rinsho Shinkeigaku. 2010 Nov;50(11):788-93. doi: 10.5692/clinicalneurol.50.788. Rinsho Shinkeigaku. 2010. PMID: 21921443 Review. Japanese.
-
Astrocytopathy in Balo's disease.Mult Scler. 2011 Jul;17(7):771-9. doi: 10.1177/1352458511400475. Epub 2011 Apr 1. Mult Scler. 2011. PMID: 21459811 Review.
-
Early disruption of glial communication via connexin gap junction in multiple sclerosis, Baló's disease and neuromyelitis optica.Neuropathology. 2015 Oct;35(5):469-80. doi: 10.1111/neup.12211. Epub 2015 May 28. Neuropathology. 2015. PMID: 26016402 Review.
-
Regulation of microglial TMEM119 and P2RY12 immunoreactivity in multiple sclerosis white and grey matter lesions is dependent on their inflammatory environment.Acta Neuropathol Commun. 2019 Dec 11;7(1):206. doi: 10.1186/s40478-019-0850-z. Acta Neuropathol Commun. 2019. PMID: 31829283 Free PMC article.
-
Reappraisal of aquaporin-4 astrocytopathy in Asian neuromyelitis optica and multiple sclerosis patients.Brain Pathol. 2011 Sep;21(5):516-32. doi: 10.1111/j.1750-3639.2011.00475.x. Epub 2011 Feb 14. Brain Pathol. 2011. PMID: 21241398 Free PMC article.
Cited by
-
Immunopathology of Tumefactive Demyelinating Lesions-From Idiopathic to Drug-Related Cases.Front Neurol. 2022 Mar 15;13:868525. doi: 10.3389/fneur.2022.868525. eCollection 2022. Front Neurol. 2022. PMID: 35418930 Free PMC article. Review.
-
Central nervous system tumefactive demyelinating lesions: Risk factors of relapse and follow-up observations.Front Immunol. 2022 Dec 1;13:1052678. doi: 10.3389/fimmu.2022.1052678. eCollection 2022. Front Immunol. 2022. PMID: 36532021 Free PMC article.
-
Microglia/Macrophages in Autoimmune Demyelinating Encephalomyelitis (Multiple Sclerosis/Neuromyelitis Optica).Int J Mol Sci. 2025 Apr 10;26(8):3585. doi: 10.3390/ijms26083585. Int J Mol Sci. 2025. PMID: 40332086 Free PMC article. Review.
-
Specific myeloid signatures in peripheral blood differentiate active and rare clinical phenotypes of multiple sclerosis.Front Immunol. 2023 Jan 25;14:1071623. doi: 10.3389/fimmu.2023.1071623. eCollection 2023. Front Immunol. 2023. PMID: 36761741 Free PMC article.
-
Modulation of microglial metabolism facilitates regeneration in demyelination.iScience. 2023 Apr 11;26(5):106588. doi: 10.1016/j.isci.2023.106588. eCollection 2023 May 19. iScience. 2023. PMID: 37138776 Free PMC article.
References
-
- Bruck W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, Lassmann H (1995) Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 38:788–796. - PubMed
-
- Burant CF, Takeda J, Brot‐Laroche E, Bell GI, Davidson NO (1992) Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem 267:14523–14526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- H26-Nanchitou(Nan)-Ippan-074/the Ministry of Health, Labour and Welfare, Japan
- 16K09694/JSPS KAKENHI Grants-in-Aid for Scientific Research (C)
- 18ek0109308h0001/the Japan Agency for Medical Research and Development (AMED)
- 19H01045/JSPS KAKENHI Grants-in-Aid for Scientific Research (A)
- 25117001/a "Glial assembly" Grant-in-Aid for Scientific Research on Innovative Areas
LinkOut - more resources
Full Text Sources
Research Materials

