Conjugated Nanohoops Incorporating Donor, Acceptor, Hetero- or Polycyclic Aromatics
- PMID: 32902109
- PMCID: PMC9542246
- DOI: 10.1002/anie.202007024
Conjugated Nanohoops Incorporating Donor, Acceptor, Hetero- or Polycyclic Aromatics
Abstract
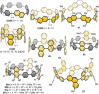
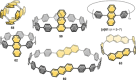
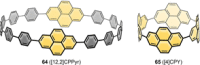
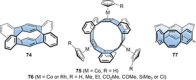
In the last 13 years several synthetic strategies were developed that provide access to [n]cycloparaphenylenes ([n]CPPs) and related conjugated nanohoops. A number of potential applications emerged, including optoelectronic devices, and their use as templates for carbon nanomaterials and in supramolecular chemistry. To tune the structural or optoelectronic properties of carbon nanohoops beyond the size-dependent effect known for [n]CPPs, a variety of aromatic rings other than benzene were introduced. In this Review, we provide an overview of the syntheses, properties, and applications of conjugated nanohoops beyond [n]CPPs with intrinsic donor/acceptor structure or such that contain acceptor, donor, heteroaromatic or polycyclic aromatic units within the hoop as well as conjugated nanobelts.
Keywords: cyclacenes; cycloparaphenylenes; macrocycles; nanohoops; optoelectronic properties.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





















References
-
- Herges R., Modern Cyclophane Chemistry, Wiley-VCH, Weinheim, 2004.
-
- Gleiter R., Haberhauer G., Aromaticity and Other Conjugation Effects, Wiley-VCH, Weinheim, 2012.
-
- Eisenberg D., Shenhar R., Rabinovitz M., Chem. Soc. Rev. 2010, 39, 2879. - PubMed
-
- Tahara K., Tobe Y., Chem. Rev. 2006, 106, 5274–5290. - PubMed
-
- Schröder A., Mekelburger H.-B., Vögtle F., in Cyclophanes (Ed.: Weber E.), Springer-Verlag, Berlin/Heidelberg, 1994, pp. 179–201.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

