Connecting the conformational behavior of cyclic octadepsipeptides with their ionophoric property and membrane permeability
- PMID: 32902550
- PMCID: PMC7796559
- DOI: 10.1039/d0ob01447h
Connecting the conformational behavior of cyclic octadepsipeptides with their ionophoric property and membrane permeability
Abstract
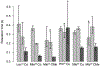
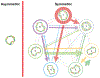
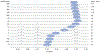
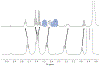
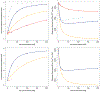
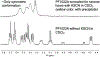
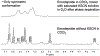
Cyclic octadepsipeptides such as PF1022A and its synthetic derivative emodepside exhibit anthelmintic activity with the latter sold as a commercial drug treatment against gastrointestinal nematodes for animal health use. The structure-permeability relationship of these cyclic depsipeptides that could ultimately provide insights into the compound bioavailability is not yet well understood. The fully N-methylated amide backbone and apolar sidechain residues do not allow for the formation of intramolecular hydrogen bonds, normally observed in the membrane-permeable conformations of cyclic peptides. Hence, any understanding gained on these depsipeptides would serve as a prototype for future design strategies. In previous nuclear magnetic resonance (NMR) studies, two macrocyclic core conformers of emodepside were detected, one with all backbone amides in trans-configuration (hereon referred as the symmetric conformer) and the other with one amide in cis-configuration (hereon referred as the asymmetric conformer). In addition, these depsipeptides were also reported to be ionophores with a preference of potassium over sodium. In this study, we relate the conformational behavior of PF1022A, emodepside, and closely related analogs with their ionophoric characteristic probed using NMR and molecular dynamics (MD) simulations and finally evaluated their passive membrane permeability using PAMPA. We find that the equilibrium between the two core conformers shifts more towards the symmetric conformer upon addition of monovalent cations with selectivity for potassium over sodium. Both the NMR experiments and the theoretical Markov state models based on extensive MD simulations indicate a more rigid backbone for the asymmetric conformation, whereas the symmetric conformation shows greater flexibility. The experimental results further advocate for the symmetric conformation binding the cation. The PAMPA results suggest that the investigated depsipeptides are retained in the membrane, which may be advantageous for the likely target, a membrane-bound potassium channel.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures
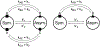


Similar articles
-
Cyclooctadepsipeptides--an anthelmintically active class of compounds exhibiting a novel mode of action.Int J Antimicrob Agents. 2003 Sep;22(3):318-31. doi: 10.1016/s0924-8579(03)00219-x. Int J Antimicrob Agents. 2003. PMID: 13678839 Review.
-
Synthesis and anthelmintic activity of thioamide analogues of cyclic octadepsipeptides such as PF1022A.Pest Manag Sci. 2001 Nov;57(11):1000-6. doi: 10.1002/ps.382. Pest Manag Sci. 2001. PMID: 11721515
-
The effect of conformation of the acyloxyalkoxy-based cyclic prodrugs of opioid peptides on their membrane permeability.J Pept Res. 1999 Apr;53(4):403-13. doi: 10.1034/j.1399-3011.1999.00077.x. J Pept Res. 1999. PMID: 10406218
-
Influence of the cyclooctadepsipeptides PF1022A and PF1022E as natural products on the design of semi-synthetic anthelmintics such as emodepside.Parasitol Res. 2005 Oct;97 Suppl 1:S11-S16. doi: 10.1007/s00436-005-1439-y. Parasitol Res. 2005. PMID: 16228266
-
Solution cis-Proline Conformation of IPCs Inhibitor Aureobasidin A Elucidated via NMR-Based Conformational Analysis.J Nat Prod. 2022 Jun 24;85(6):1449-1458. doi: 10.1021/acs.jnatprod.1c01071. Epub 2022 May 27. J Nat Prod. 2022. PMID: 35622967 Review.
Cited by
-
The backbone constitution drives passive permeability independent of side chains in depsipeptide and peptide macrocycles inspired by ent-verticilide.Chem Sci. 2024 Aug 15;15(36):14977-87. doi: 10.1039/d4sc02758b. Online ahead of print. Chem Sci. 2024. PMID: 39211739 Free PMC article.
-
Mixture-Based Screening of Focused Combinatorial Libraries by NMR: Application to the Antiapoptotic Protein hMcl-1.J Med Chem. 2023 Jul 27;66(14):10108-10118. doi: 10.1021/acs.jmedchem.3c01073. Epub 2023 Jul 18. J Med Chem. 2023. PMID: 37464766 Free PMC article.
-
Macrocycle-Antibiotic Hybrids: A Path to Clinical Candidates.Front Chem. 2021 Apr 30;9:659845. doi: 10.3389/fchem.2021.659845. eCollection 2021. Front Chem. 2021. PMID: 33996753 Free PMC article. Review.
-
A Bidirectional Permeability Assay for beyond Rule of 5 Compounds.Pharmaceutics. 2021 Jul 27;13(8):1146. doi: 10.3390/pharmaceutics13081146. Pharmaceutics. 2021. PMID: 34452112 Free PMC article.
-
Amide-to-ester substitution as a stable alternative to N-methylation for increasing membrane permeability in cyclic peptides.Nat Commun. 2023 Mar 17;14(1):1416. doi: 10.1038/s41467-023-36978-z. Nat Commun. 2023. PMID: 36932083 Free PMC article.
References
-
- Nielsen DS, Shepherd NE, Xu W, Lucke AJ, Stoermer MJ and Fairlie DP, Chem. Rev, 2017, 117, 8094–8128. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

