Clinical pre-test probability for obstructive coronary artery disease: insights from the European DISCHARGE pilot study
- PMID: 32902743
- PMCID: PMC7880945
- DOI: 10.1007/s00330-020-07175-z
Clinical pre-test probability for obstructive coronary artery disease: insights from the European DISCHARGE pilot study
Abstract
Objectives: To test the accuracy of clinical pre-test probability (PTP) for prediction of obstructive coronary artery disease (CAD) in a pan-European setting.
Methods: Patients with suspected CAD and stable chest pain who were clinically referred for invasive coronary angiography (ICA) or computed tomography (CT) were included by clinical sites participating in the pilot study of the European multi-centre DISCHARGE trial. PTP of CAD was determined using the Diamond-Forrester (D+F) prediction model initially introduced in 1979 and the updated D+F model from 2011. Obstructive coronary artery disease (CAD) was defined by one at least 50% diameter coronary stenosis by both CT and ICA.
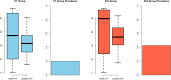
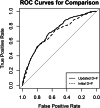
Results: In total, 1440 patients (654 female, 786 male) were included at 25 clinical sites from May 2014 until July 2017. Of these patients, 725 underwent CT, while 715 underwent ICA. Both prediction models overestimated the prevalence of obstructive CAD (31.7%, 456 of 1440 patients, PTP: initial D+F 58.9% (28.1-90.6%), updated D+F 47.3% (34.2-59.9%), both p < 0.001), but overestimation of disease prevalence was higher for the initial D+F (p < 0.001). The discriminative ability was higher for the updated D+F 2011 (AUC of 0.73 95% confidence interval [CI] 0.70-0.76 versus AUC of 0.70 CI 0.67-0.73 for the initial D+F; p < 0.001; odds ratio (or) 1.55 CI 1.29-1.86, net reclassification index 0.11 CI 0.05-0.16, p < 0.001).
Conclusions: Clinical PTP calculation using the initial and updated D+F prediction models relevantly overestimates the actual prevalence of obstructive CAD in patients with stable chest pain clinically referred for ICA and CT suggesting that further refinements to improve clinical decision-making are needed.
Trial registration: https://www.clinicaltrials.gov/ct2/show/NCT02400229 KEY POINTS: • Clinical pre-test probability calculation using the initial and updated D+F model overestimates the prevalence of obstructive CAD identified by ICA and CT. • Overestimation of disease prevalence is higher for the initial D+F compared with the updated D+F. • Diagnostic accuracy of PTP assessment varies strongly between different clinical sites throughout Europe.
Keywords: Computed tomography angiography; Coronary artery disease; Prevalence; Probability of disease.
Conflict of interest statement
Sarah Feger: Dr. Feger reports grants from European Commission, other from Vital Images, other from AG Mednet, during the conduct of the study; grants from Siemens Medical Solutions; grants from GE Healthcare; grants from Toshiba Medical Systems; and grants from Philips Medical Systems, outside the submitted work.
Paolo Ibes: Dr. Ibes reports grants and other from European Commission, from Vital Images, and from AG Mednet, during the conduct of the study; grants from Siemens Medical Solutions; grants from GE Healthcare; grants from Toshiba Medical Systems; and grants from Philips Medical Systems, outside the submitted work.
Adriane E. Napp: Dr. Napp reports grants from European Commission, other from Vital Images, and other from AG Mednet, during the conduct of the study; grants from Siemens Medical Solutions; grants from GE Healthcare; grants from Toshiba Medical Systems; and grants from Philips Medical Systems, outside the submitted work.
Alexander Lembcke: Dr. Lembcke reports grants from European Commission, other from Vital Images, and other from AG Mednet, during the conduct of the study; grants from Siemens Medical Solutions; grants from GE Healthcare; grants from Toshiba Medical Systems; grants from Philips Medical Systems, and personal fees from PAREXEL International GmbH, outside the submitted work.
Michael Laule: Dr. Laule has nothing to disclose.
Henryk Dreger: Dr. Dreger has nothing to disclose.
Björn Bokelmann: Mr. Bokelmann reports grants from European Commission, other from Vital Images, and other from AG Mednet, during the conduct of the study; grants from Siemens Medical Solutions; grants from GE Healthcare; grants from Toshiba Medical Systems; and grants from Philips Medical Systems, outside the submitted work.
Gershan K. Davis: Dr. Davis reports grants from the European Commission, during the conduct of the study.
Giles Roditi: Dr. Roditi reports grants from the European Commission, during the conduct of the study.
Ignacio Diez: Dr. Diez Gonzalez reports grants from the European Commission, during the conduct of the study.
Stephen Schröder: Dr. Schröder reports grants from the European Commission, during the conduct of the study.
Fabian Plank: Dr. Plank reports grants from the European Commission, during the conduct of the study.
Pal Maurovich-Horvat: Dr. Maurovich-Horvat reports grants from the European Commission, during the conduct of the study.
Radosav Vidakovic: Dr. Vidakovic reports grants from the European Commission, during the conduct of the study.
Josef Veselka: Dr. Veselka reports grants from the European Commission, during the conduct of the study.
Malgorzata Ilnicka-Suckiel: Dr. Ilnicka-Suckiel reports grants from the European Commission, during the conduct of the study.
Andrejs Erglis: Dr. Erglis reports grants from European Commission, during the conduct of the study; grants from Abbott Vascular; grants from Boston Scientific; personal fees from Abbott Vascular; personal fees from Boston Scientific; personal fees from Biotronik; personal fees from Biosensors; personal fees from Cordis; and personal fees from Medtronik, outside the submitted work.
Teodora Benedek: Dr. Benedek reports grants from European Commission, during the conduct of the study; grants from Romanian Ministry of European Funds; the Romanian Government and the European Union; and grants from Romanian Ministry of European Funds, the Romanian Government, and the European Union, outside the submitted work.
José Rodriguez-Palomares: Dr. Rodriguez-Palomares reports grants from the European Commission, during the conduct of the study.
Luca Saba: Dr. Saba reports grants from the European Commission, during the conduct of the study; personal fees from Springer; and personal fees from Francis and Taylor, outside the submitted work.
Klaus Kofoed: Dr. Fuglsang Kofoed reports grants from European Commission, during the conduct of the study; grants from AP Møller og hustru Chastine McKinney Møllers Fond; grants from The John and Birthe Meyer Foundation; grants from Research Council of Rigshopitalet; grants from The University of Copenhagen; grants from The Danish Heart Foundation; grants from The Lundbeck Foundation; grants from The Danish Agency for Science, Technology and Innovation by The Danish Council for Strategic Research; grants from CATCH-2 trial; grants from CSub320 trial; and grants from Toshiba Medical Corporation, other from Toshiba Medical Corporation, outside the submitted work.
Matthias Gutberlet: Dr. Gutberlet reports grants from European Commission, during the conduct of the study; grants from Bayer Healthcare; grants and other from Bayer Healthcare; grants from Bracco; and grants from Philips; other from Philips Medical Systems, from Siemens Medical Solutions, other from Siemens Medical Solutions, outside the submitted work.
Filip Ađić: Dr. Ađić reports grants from the European Commission, during the conduct of the study.
Mikko Pietilä: Dr. Pietila reports grants from European Commission, during the conduct of the study; personal fees from AstraZeneca, personal fees from Novartis, personal fees from MSD, non-financial support from Bayer, and non-financial support from B. Braun, outside the submitted work.
Rita Faria: Dr. Faria reports grants from the European Commission, during the conduct of the study; other from the Portuguese Ministry of Health, outside the submitted work.
Audronė Vaitiekienė Dr. Vaitiekiene reports grants from the European Commission, during the conduct of the study.
Jonathan Dodd: Dr. Dodd reports grants from European Commission, during the conduct of the study; personal fees from co-author of the StatDx Cardiovascular textbook, 2nd Ed; other from Associate editor for Radiology, European Radiology, QJM and Respirology, outside the submitted work.
Patrick Donnelly: Dr. Donnelly reports grants from the European Commission, during the conduct of the study; grants from Southeastern Health and Social Care Trust Innovation Research Development Group Fund, outside the submitted work.
Marco Francone: Dr. Francone reports grants from the European Commission, during the conduct of the study; personal fees from Bayer Medical Imaging and personal fees from Bracco, outside the submitted work.
Cezary Kepka: Dr. Kepka reports grants from the European Commission, during the conduct of the study.
Balazs Ruzsics: Dr. Ruzsics reports grants from the European Commission, during the conduct of the study.
Jacqueline Müller-Nordhorn: Dr. Müller-Nordhorn reports grants from the European Commission, during the conduct of the study.
Peter Schlattmann: Dr. Schlattmann reports grants from the European Commission, during the conduct of the study.
Marc Dewey: Dr. Dewey reports grants from the European Commission, other from Vital Images, other from AG Mednet, during the conduct of the study; grants from Siemens Medical Solutions; grants from GE Healthcare; grants from Toshiba Medical Systems; grants from Philips Medical Systems; and grants from Heisenberg Programme of DFG, outside the submitted work.
Figures


References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

