Telemetry-controlled simultaneous stimulation-and-recording device (SRD) to study interhemispheric cortical circuits in rat primary somatosensory (SI) cortex
- PMID: 32903340
- PMCID: PMC7422589
- DOI: 10.1186/s42490-019-0019-7
Telemetry-controlled simultaneous stimulation-and-recording device (SRD) to study interhemispheric cortical circuits in rat primary somatosensory (SI) cortex
Abstract
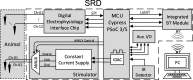
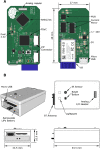
Background: A growing need exists for neuroscience platforms that can perform simultaneous chronic recording and stimulation of neural tissue in animal models in a telemetry-controlled fashion with signal processing for analysis of the chronic recording data and external triggering capability. We describe the system design, testing, evaluation, and implementation of a wireless simultaneous stimulation-and-recording device (SRD) for modulating cortical circuits in physiologically identified sites in primary somatosensory (SI) cortex in awake-behaving and freely-moving rats. The SRD was developed using low-cost electronic components and open-source software. The function of the SRD was assessed by bench and in-vivo testing.
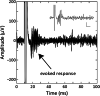
Results: The SRD recorded spontaneous spiking and bursting neuronal activity, evoked responses to programmed intracortical microstimulation (ICMS) delivered internally by the SRD, and evoked responses to external peripheral forelimb stimulation.
Conclusions: The SRD is capable of wireless stimulation and recording on a predetermined schedule or can be wirelessly synchronized with external input as would be required in behavioral testing prior to, during, and following ICMS.
Keywords: Brain-computer interface; Intracortical microstimulation; Primary somatosensory cortex.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
