Physiological and Biochemical Mechanisms and Cytology of Cold Tolerance in Brassica napus
- PMID: 32903421
- PMCID: PMC7434931
- DOI: 10.3389/fpls.2020.01241
Physiological and Biochemical Mechanisms and Cytology of Cold Tolerance in Brassica napus
Abstract
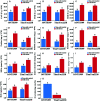
Cold damage has negatively impacted the yield, growth and quality of the edible cooking oil in Northern China and Brassica napus L.(rapeseed) planting areas decreased because of cold damage. In the present study we analyzed two Brassica napus cultivars of 16NTS309 (highly resistant to cold damage) and Tianyou2238 (cold sensitive) from Gansu Province, China using physiological, biochemical and cytological methods to investigate the plant's response to cold stress. The results showed that cold stress caused seedling dehydration, and the contents of malondialdehyde (MDA), relative electrolyte leakage and O2 - and H2O2 were increased in Tianyou2238 than 16NTS309 under cold stress at 4°C for 48 h, as well as the proline, soluble protein and soluble sugars markedly accumulated, and antioxidant enzymes of peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT) were higher in 16NTS309 compared with in Tianyou2238, which play key roles in prevention of cell damage. After exposure to cold stress, the accumulation of the blue formazan precipitate and reddish brown precipitate indicated that O2 - and H2O2, respectively, were produced in the root, stem, and leaf were higher than under non-cold conditions. Contents of O2 - and H2O2 in cultivar Tianyou2238 were higher than 16NTS309, this is consistent with the phenotypic result. To understand the specific distribution of O2 - in the sub-cellular, we found that in both cultivars O2 - signals were distributed mainly in cambium tissue, meristematic cells, mesophyll cytoplasm, and surrounding the cell walls of root, stem, leaves, and leaf vein by morphoanatomical analysis, but the quantities varied. Cold stress also triggered obvious ultrastructural alterations in leaf mesophyll of Tianyou2238 including the damage of membrane system, destruction of chloroplast and swelling of mitochondria. This study are useful to provide new insights about the physiological and biochemical mechanisms and cytology associated with the response of B. napus to cold stress for use in breeding cold-resistant varieties.
Keywords: Brassica napus; biochemical index; cold stress; cytology; physiological index; super-oxide anion (O2−).
Copyright © 2020 Qi, Wang, Ma, Qi, Liu, Chen, Wu, Wang, Yang, Wu and Sun.
Figures











Similar articles
-
Oxidative injury and antioxidant enzymes regulation in arsenic-exposed seedlings of four Brassica napus L. cultivars.Environ Sci Pollut Res Int. 2015 Jul;22(14):10699-712. doi: 10.1007/s11356-015-4269-1. Epub 2015 Mar 11. Environ Sci Pollut Res Int. 2015. PMID: 25752633
-
Cadmium stress alters the redox reaction and hormone balance in oilseed rape (Brassica napus L.) leaves.Environ Sci Pollut Res Int. 2016 Feb;23(4):3758-69. doi: 10.1007/s11356-015-5640-y. Epub 2015 Oct 24. Environ Sci Pollut Res Int. 2016. PMID: 26498815
-
Synergistic effects of chromium and copper on photosynthetic inhibition, subcellular distribution, and related gene expression in Brassica napus cultivars.Environ Sci Pollut Res Int. 2019 Apr;26(12):11827-11845. doi: 10.1007/s11356-019-04450-5. Epub 2019 Feb 28. Environ Sci Pollut Res Int. 2019. PMID: 30820917
-
Transcriptome Profile Analysis of Winter Rapeseed (Brassica napus L.) in Response to Freezing Stress, Reveal Potentially Connected Events to Freezing Stress.Int J Mol Sci. 2019 Jun 5;20(11):2771. doi: 10.3390/ijms20112771. Int J Mol Sci. 2019. PMID: 31195741 Free PMC article.
-
[Effects of chilling stress on antioxidant system and ultrastructure of walnut cultivars].Ying Yong Sheng Tai Xue Bao. 2015 May;26(5):1320-6. Ying Yong Sheng Tai Xue Bao. 2015. PMID: 26571647 Chinese.
Cited by
-
Transcriptome Profiling of Stem-Differentiating Xylem in Response to Abiotic Stresses Based on Hybrid Sequencing in Cunninghamia lanceolata.Int J Mol Sci. 2022 Nov 12;23(22):13986. doi: 10.3390/ijms232213986. Int J Mol Sci. 2022. PMID: 36430463 Free PMC article.
-
Transcriptomic Analysis Reveals Potential Gene Regulatory Networks Under Cold Stress of Loquat (Eriobotrya japonica Lindl.).Front Plant Sci. 2022 Jul 22;13:944269. doi: 10.3389/fpls.2022.944269. eCollection 2022. Front Plant Sci. 2022. PMID: 35937353 Free PMC article.
-
Integrated methylome and transcriptome analysis unravel the cold tolerance mechanism in winter rapeseed(Brassica napus L.).BMC Plant Biol. 2022 Aug 26;22(1):414. doi: 10.1186/s12870-022-03797-1. BMC Plant Biol. 2022. PMID: 36008781 Free PMC article.
-
Functional Characterization of MaZIP4, a Gene Regulating Copper Stress Tolerance in Mulberry (Morus atropurpurea R.).Life (Basel). 2022 Aug 26;12(9):1311. doi: 10.3390/life12091311. Life (Basel). 2022. PMID: 36143348 Free PMC article.
-
Phosphoproteomics of cold stress-responsive mechanisms in Rhododendron chrysanthum.Mol Biol Rep. 2022 Jan;49(1):303-312. doi: 10.1007/s11033-021-06874-0. Epub 2021 Nov 6. Mol Biol Rep. 2022. PMID: 34743272
References
-
- Ali S., Gill R., Mwamba T., Zhang N., Lv M., Ulhassan Z., et al. (2018. a). Differential cobalt-induced effects on plant growth, ultrastructural modifications, and antioxidative response among four Brassica napus (L.) cultivars. Int. J. Environ. Sci. Technol. (Tehran) 15, 2685–2700. 10.1007/s13762-017-1629-z - DOI
-
- Asada K., Takahashl M. (1987). “Production and scavenging of active oxygen in photosynthesis,” in Photoinhibition. Eds. Kyle D. J., Osmond C. B., Arntzen C. J. (Amsterdam, FL: Elsevier Science Publishers; ), 227–287.
-
- Bajji M., Kinet J.-M., Lutts S. (2002). The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 36, 61–70. 10.1023/A:1014732714549 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

