Soybean Embryonic Axis Transformation: Combining Biolistic and Agrobacterium-Mediated Protocols to Overcome Typical Complications of In Vitro Plant Regeneration
- PMID: 32903423
- PMCID: PMC7434976
- DOI: 10.3389/fpls.2020.01228
Soybean Embryonic Axis Transformation: Combining Biolistic and Agrobacterium-Mediated Protocols to Overcome Typical Complications of In Vitro Plant Regeneration
Abstract
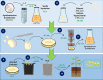
The first successful attempt to generate genetically modified plants expressing a transgene was preformed via T-DNA-based gene transfer employing Agrobacterium tumefaciens-mediated genetic transformation. Limitations over infectivity and in vitro tissue culture led to the development of other DNA delivery systems, such as the biolistic method. Herein, we developed a new one-step protocol for transgenic soybean recovery by combining the two different transformation methods. This protocol comprises the following steps: agrobacterial preparation, seed sterilization, soybean embryo excision, shoot-cell injury by tungsten-microparticle bombardment, A. tumefaciens-mediated transformation, embryo co-cultivation in vitro, and selection of transgenic plants. This protocol can be completed in approximately 30-40 weeks. The average efficiency of producing transgenic soybean germlines using this protocol was 9.84%, similar to other previously described protocols. However, we introduced a more cost-effective, more straightforward and shorter methodology for transgenic plant recovery, which allows co-cultivation and plant regeneration in a single step, decreasing the chances of contamination and making the manipulation easier. Finally, as a hallmark, our protocol does not generate plant chimeras, in contrast to traditional plant regeneration protocols applied in other Agrobacterium-mediated transformation methods. Therefore, this new approach of plant transformation is applicable for studies of gene function and the production of transgenic cultivars carrying different traits for precision-breeding programs.
Keywords: Agrobacterium-mediated transformation; Glycine max; embryonic axis; genetic transformation; high-efficiency plant transformation; particle bombardment.
Copyright © 2020 Paes de Melo, Lourenço-Tessutti, Morgante, Santos, Pinheiro, de Jesus Lins, Silva, Macedo, Fontes and Grossi-de-Sa.
Figures





References
-
- Bailey M. A., Boerma H. R., Parrott W. A. (1993). Genotype effects on proliferative embryogenesis and plant regeneration of soybean. Vitro Cell Dev. Biol. Plant 29, 102–108. 10.1007/BF02632279 - DOI
-
- Droste A., Pasquali G., Bodanese-Zanettini M. H. (2000). Integrated bombardment and Agrobacterium transformation system: An alternative method for soybean transformation. Plant Mol. Biol. Rep. 18, 51–59. 10.1007/BF02825294 - DOI
-
- Dutta Gupta S. (2010). “Role of free radicals and antioxidants in in vitro morphogenesis,” in Reactive Oxygen Species and Antioxidants in Higher Plants, (USA: CRC Press and Sci. Pub.) 229–247.
-
- Gao X., Zhang Y., He Z., Fu X. (2017). “Gibberellins,” in Hormone Metabolism and Signaling in Plants (Cambrigde, USA: Elsevier; ), 107–160. 10.1016/B978-0-12-811562-6.00004-9 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

