Placental Microbial Colonization and Its Association With Pre-eclampsia
- PMID: 32903432
- PMCID: PMC7434969
- DOI: 10.3389/fcimb.2020.00413
Placental Microbial Colonization and Its Association With Pre-eclampsia
Abstract
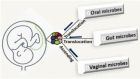
The existence and role of the microbiome in regulating physiological and pathophysiological conditions including metabolism, energy homeostasis, immune tolerance, behavior, obesity, diabetes, and cardiovascular-related diseases is of immense interest. It is now clear that the human placenta is not sterile, but rather colonized with microbes. The placental and vaginal microbiomes are distinct however, the placental microbiome is comparable with the oral microbiome, with a limited variation when compared with the gut microbiome. Pre-eclampsia (PE), a pregnancy-specific hypertensive disorder, remains the leading cause of maternal-fetal morbidity and mortality. This is largely due to the lack of a clear etiology of PE and consequently, diagnostic strategies, and treatment are sub-optimal. The present review focuses on the current understanding of the placental microbiome and its implication in the etiology of PE. It provides a perspective on the alteration of placental microbiome as a possible therapeutic approach in the prevention and management of PE.
Keywords: immune tolerance; metabolism; microbe; placental microbiome; pre-eclampsia; pregnancy.
Copyright © 2020 Olaniyi, Moodley, Mahabeer and Mackraj.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

