The Protective Effect of Luteolin in Glucocorticoid-Induced Osteonecrosis of the Femoral Head
- PMID: 32903480
- PMCID: PMC7435053
- DOI: 10.3389/fphar.2020.01195
The Protective Effect of Luteolin in Glucocorticoid-Induced Osteonecrosis of the Femoral Head
Abstract
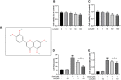
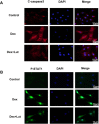
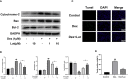
Glucocorticoid-induced osteonecrosis of the femoral head (GIONFH) is a frequently occurring type of nontraumatic osteonecrosis. A failure of the timely treatment can eventually result in the collapse of the subchondral bone structure. Luteolin (Lut), a compound extracted from Rhizoma Drynariae, is reported to possess multiple pharmacological properties including anticancer, antioxidant, antiapoptosis, and antiinflammatory properties. However, whether Lut has a protective effect on the development of GIONFH remains unclear. In this study, we evaluated the effect of Lut on Dexamethasone (Dex)-induced STAT1/caspase3 pathway in vitro and evaluated GIONFH model in vivo. In vitro, Lut inhibited the upregulation of Dex-induced phospho-STAT1, cleaved caspase9, and cleaved caspase3. In addition, Lut inhibited Dex-induced expression of Bax and cytochrome c and increased the expression of B cell lymphoma-2(Bcl-2). In vivo, Lut decreased the proportion of empty lacunae in rats with GIONFH. Taken together, these findings indicate that Lut may have therapeutic potential in the treatment of GIONFH. Further, this effect might be achieved by suppressing mitochondrial apoptosis of osteoblasts via inhibition of STAT1 activity.
Keywords: STAT1/caspase3 pathway; apoptosis; glucocorticoid-induced osteonecrosis of the femoral head; luteolin; mitochondrial pathway.
Copyright © 2020 Yan, Zhan, Qi, Lin, Huang, Xue and Pan.
Figures




Similar articles
-
Asiatic acid prevents glucocorticoid-induced femoral head osteonecrosis via PI3K/AKT pathway.Int Immunopharmacol. 2024 Mar 30;130:111758. doi: 10.1016/j.intimp.2024.111758. Epub 2024 Feb 28. Int Immunopharmacol. 2024. PMID: 38422771
-
Luteolin ameliorates necroptosis in Glucocorticoid-induced osteonecrosis of the femoral head via RIPK1/RIPK3/MLKL pathway based on network pharmacology analysis.Biochem Biophys Res Commun. 2023 Jun 18;661:108-118. doi: 10.1016/j.bbrc.2023.04.023. Epub 2023 Apr 11. Biochem Biophys Res Commun. 2023. PMID: 37099894
-
Astaxanthin-mediated Nrf2 activation ameliorates glucocorticoid-induced oxidative stress and mitochondrial dysfunction and impaired bone formation of glucocorticoid-induced osteonecrosis of the femoral head in rats.J Orthop Surg Res. 2024 May 14;19(1):294. doi: 10.1186/s13018-024-04775-z. J Orthop Surg Res. 2024. PMID: 38745231 Free PMC article.
-
The role of biomechanical forces and MALAT1/miR-329-5p/PRIP signalling on glucocorticoid-induced osteonecrosis of the femoral head.J Cell Mol Med. 2021 Jun;25(11):5164-5176. doi: 10.1111/jcmm.16510. Epub 2021 May 3. J Cell Mol Med. 2021. PMID: 33939272 Free PMC article.
-
Luteolin: A Flavonoid that Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms.Front Pharmacol. 2017 Oct 6;8:692. doi: 10.3389/fphar.2017.00692. eCollection 2017. Front Pharmacol. 2017. PMID: 29056912 Free PMC article. Review.
Cited by
-
Anti-Osteoporosis Effect of Perilla frutescens Leaf Hexane Fraction through Regulating Osteoclast and Osteoblast Differentiation.Molecules. 2022 Jan 26;27(3):824. doi: 10.3390/molecules27030824. Molecules. 2022. PMID: 35164085 Free PMC article.
-
Mitochondrial maintenance as a novel target for treating steroid-induced osteonecrosis of femoral head: a narrative review.EFORT Open Rev. 2024 Nov 8;9(11):1013-1022. doi: 10.1530/EOR-24-0023. EFORT Open Rev. 2024. PMID: 39513701 Free PMC article. Review.
-
Effect of femoral head necrosis cystic area on femoral head collapse and stress distribution in femoral head: A clinical and finite element study.Open Med (Wars). 2022 Jul 13;17(1):1282-1291. doi: 10.1515/med-2022-0506. eCollection 2022. Open Med (Wars). 2022. PMID: 35892078 Free PMC article.
-
Cyasterone has a protective effect on steroid-induced Osteonecrosis of the femoral head.PLoS One. 2023 Oct 30;18(10):e0293530. doi: 10.1371/journal.pone.0293530. eCollection 2023. PLoS One. 2023. PMID: 37903142 Free PMC article.
-
Tanshinone I Mitigates Steroid-Induced Osteonecrosis of the Femoral Head and Activates the Nrf2 Signaling Pathway in Rats.Evid Based Complement Alternat Med. 2021 Dec 31;2021:8002161. doi: 10.1155/2021/8002161. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 35111227 Free PMC article.
References
-
- Al-Megrin W. A., Alkhuriji A. F., Yousef A. O. S., Metwally D. M., Habotta O. A., Kassab R. B., et al. (2019). Antagonistic Efficacy of Luteolin against Lead Acetate Exposure-Associated with Hepatotoxicity is Mediated via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities. Antioxid. (Basel) 9, 10. 10.3390/antiox9010010 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

