Use of Cell and Genome Modification Technologies to Generate Improved "Off-the-Shelf" CAR T and CAR NK Cells
- PMID: 32903482
- PMCID: PMC7438733
- DOI: 10.3389/fimmu.2020.01965
Use of Cell and Genome Modification Technologies to Generate Improved "Off-the-Shelf" CAR T and CAR NK Cells
Abstract
The broad success of adoptive immunotherapy to treat human cancer has resulted in a paradigm shift in modern medicine. Modification of autologous and allogenic immune cells with chimeric antigen receptors (CAR) designed to target specific antigens on tumor cells has led to production of CAR T and CAR NK cell therapies, which are ever more commonly introduced into cancer patient treatment protocols. While allogenic T cells may offer advantages such as improved anti-tumor activity, they also carry the risk of adverse reactions like graft-versus-host disease. This risk can be mitigated by use of autologous immune cells, however, the time needed for T and/or NK cell isolation, modification and expansion may be too long for some patients. Thus, there is an urgent need for strategies to robustly produce "off-the-shelf" CAR T and CAR NK cells, which could be used as a bridging therapy between cancer diagnosis or relapse and allogeneic transplantation. Advances in genome modification technologies have accelerated the generation of designer cell therapy products, including development of "off-the-shelf" CAR T cells for cancer immunotherapy. The feasibility and safety of such approaches is currently tested in clinical trials. This review will describe cell sources for CAR-based therapies, provide background of current genome editing techniques and the applicability of these approaches for generation of universal "off-the-shelf" CAR T and NK cell therapeutics.
Keywords: CRISPR-Cas9; T cell; chimeric antigen receptor; genome editing; immunotherapy.
Copyright © 2020 Morgan, Büning, Sauer and Schambach.
Figures
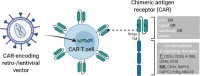

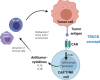
References
-
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. and et al.: graft-versus-leukemia reactions after bone marrow transplantation. Blood. (1990) 75:555–62. - PubMed
-
- Samelson LE, Donovan JA, Isakov N, Ota Y, Wange RL. Signal transduction mediated by the T-cell antigen receptor. Ann N Y Acad Sci. (1995) 766:157–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

