Bases for Treating Skin Aging With Artificial Mitochondrial Transfer/Transplant (AMT/T)
- PMID: 32903493
- PMCID: PMC7438394
- DOI: 10.3389/fbioe.2020.00919
Bases for Treating Skin Aging With Artificial Mitochondrial Transfer/Transplant (AMT/T)
Abstract
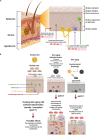
The perception of mitochondria as only the powerhouse of the cell has dramatically changed in the last decade. It is now accepted that in addition to being essential intracellularly, mitochondria can promote cellular repair when transferred from healthy to damaged cells. The artificial mitochondria transfer/transplant (AMT/T) group of techniques emulate this naturally occurring process and have been used to develop therapies to treat a range of diseases including cardiac and neurodegenerative. Mitochondria accumulate damage with time, resulting in cellular senescence. Skin cells and its mitochondria are profoundly affected by ultraviolet radiation and other factors that induce premature and accelerated aging. In this article, we propose the basis to use AMT/T to treat skin aging by transferring healthy mitochondria to senescent cells, possibly revitalizing them. We provide insightful information about how skin structure, components, and cells could age rapidly depending on the amount of damage received. Arguments are shown in favor of the use of AMT/T to treat aging skin and its cells, among them the possibility to stop free radical production, add new genetic material, and provide an energetic boost to help cells prolong their viability over time. This article intends to present one of the many aspects in which mitochondria could be used as a universal treatment for cell and tissue damage and aging.
Keywords: MitoCeption; aging; artificial mitochondria transfer transplant (AMT/T); mitochondria; regenerative medicine; senescence; skin.
Copyright © 2020 Balcázar, Cañizares, Borja, Pontón, Bisiou, Carabasse, Bacilieri, Canavese, Diaz, Cabrera and Caicedo.
Figures
References
-
- Angelova P. R., Abramov A. Y. (2018). Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 592 692–702. - PubMed
LinkOut - more resources
Full Text Sources

