Lactobacillus johnsonii BS15 Prevents Psychological Stress-Induced Memory Dysfunction in Mice by Modulating the Gut-Brain Axis
- PMID: 32903531
- PMCID: PMC7438410
- DOI: 10.3389/fmicb.2020.01941
Lactobacillus johnsonii BS15 Prevents Psychological Stress-Induced Memory Dysfunction in Mice by Modulating the Gut-Brain Axis
Abstract
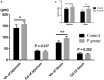
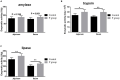
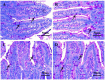
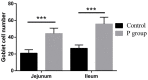
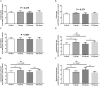
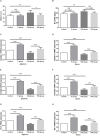
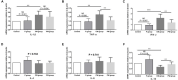
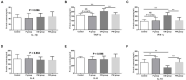
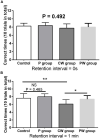
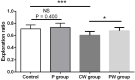
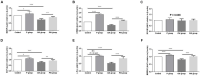
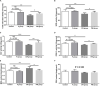
Researchers are attempting to harness the advantages of the gut-brain axis to prevent neurocognitive disorders by enhancing intestinal health. In this study, four groups of ICR mice were orally gavaged with either phosphate-buffered saline (control and CW groups) or the probiotic strain Lactobacillus johnsonii BS15 (P and PW group; daily amounts of 2 × 108 colony-forming units) for 28 days. From days 22 to 28, the mice in the CW and PW groups were subjected to water-avoidance stress (WAS). The issue of whether psychological stress-induced memory dysfunction can be prevented via L. johnsonii BS15 pretreatment to modulate the gut-brain axis was investigated. Results show that L. johnsonii BS15 enhanced gut development by increasing villus height in the jejunum and ileum as well as villus height:crypt depth ratio in the ileum. L. johnsonii BS15 increased the activities of digestive enzymes, including trypsin and lipase in the jejunum and ileum. The intestinal goblet cell number was also increased by L. johnsonii BS15 pretreatment. Moreover, L. johnsonii BS15 balanced the gut microbiota by increasing the log10 DNA gene copies of Lactobacillus spp. and L. johnsonii and decreasing that of Enterobacteriaceae in the cecum. L. johnsonii BS15 also exerted preventive effects on intestinal permeability WAS by modulating diamine oxidase and D-lactate levels in the serum and mRNA expression levels of the tight junction proteins claudin-1, occludin, and ZO-1 in the jejunum and ileum. L. johnsonii BS15 pretreatment modulated inflammatory factors, specifically tumor necrosis factor-alpha, interferon-gamma, and interleukin-10. L. johnsonii BS15 pretreatment improved their performance in two behavioral tests, namely the novel object and T-maze tests. This result indicates that psychological stress-induced memory dysfunction possibly could be prevented through the gut-brain axis. In addition, L. johnsonii BS15 exerted beneficial effects on the hippocampus by modulating memory-related functional proteins, especially those related to synaptic plasticity, such as brain-derived neurotrophic factor and stem cell factor. Moreover, L. johnsonii BS15 recovered antioxidant capacity and exerted protective effects on mitochondrion-mediated apoptosis in the hippocampus. Collectively, the modulation of the gut-brain axis by L. johnsonii BS15 could be considered a promising non-invasive treatment modality for psychological stress-induced memory dysfunction.
Keywords: Lactobacillus; gut microbiota; gut–brain axis; memory dysfunction; probiotic.
Copyright © 2020 Wang, Sun, Xin, Zhang, Sun, Ni, Zeng and Bai.
Figures
References
-
- Albert-Gasco H., Garcia-Aviles A., Moustafa S., Sanchez-Sarasua S., Gundlach A. L., Olucha-Bordonau F. E., et al. (2017). Central relaxin-3 receptor (RXFP3) activation increases ERK phosphorylation in septal cholinergic neurons and impairs spatial working memory. Brain Struct. Funct. 222 449–463. 10.1007/s00429-016-1227-8 - DOI - PubMed
-
- Bravo J. A., Forsythe P., Chew M. V., Escaravage E., Savignac H. M., Dinan T. G., et al. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U.S.A. 108 16050–16055. 10.1073/pnas.1102999108 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

