Uncovering the Mechanisms of Cryptotanshinone as a Therapeutic Agent Against Hepatocellular Carcinoma
- PMID: 32903546
- PMCID: PMC7438559
- DOI: 10.3389/fphar.2020.01264
Uncovering the Mechanisms of Cryptotanshinone as a Therapeutic Agent Against Hepatocellular Carcinoma
Abstract
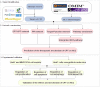
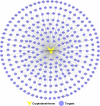
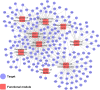
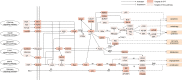
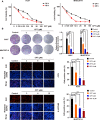
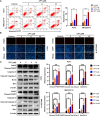
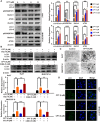
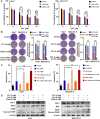
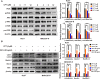
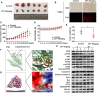
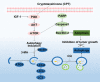
Hepatocellular carcinoma (HCC) is a fatal and dominant form of liver cancer that currently has no effective treatment or positive prognosis. In this study, we explored the antitumor effects of cryptotanshinone (CPT) against HCC and the molecular mechanisms underlying these effects using a systems pharmacology and experimental validation approach. First, we identified a total of 296 CPT targets, 239 of which were also HCC-related targets. We elucidated the mechanisms by which CPT affects HCC through multiple network analysis, including CPT-target network analysis, protein-protein interaction network analysis, target-function network analysis, and pathway enrichment analysis. In addition, we found that CPT induced apoptosis in Huh7 and MHCC97-H ells due to increased levels of cleaved PARP, Bax, and cleaved caspase-3 and decreased Bcl-2 expression. CPT also induced autophagy in HCC cells by increasing LC3-II conversion and the expression of Beclin1 and ATG5, while decreasing the expression of p62/SQSTM1. Autophagy inhibitors (3-methyladenine and chloroquine) enhanced CPT-induced proliferation and apoptosis, suggesting that CPT-induced autophagy may protect HCC cells against cell death. Furthermore, CPT was found to inhibit the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway. Interestingly, activation of PI3K by insulin-like growth factor-I inhibited CPT-induced apoptosis and autophagy, suggesting that the PI3K/AKT/mTOR signaling pathway is involved in both CPT-induced apoptosis and autophagy. Finally, CPT was found to inhibit the growth of Huh7 xenograft tumors. In conclusion, we first demonstrated the antitumor effects of CPT in Huh7 and MHCC97-H cells, both in vitro and in vivo. We elucidated the potential antitumor mechanism of CPT, which involved inducing apoptosis and autophagy by inhibiting the PI3K/Akt/mTOR signaling pathway. Our findings may provide valuable insights into the clinical application of CPT, serving as a potential candidate therapeutic agent for HCC treatment.
Keywords: apoptosis; autophagy; cryptotanshinone; hepatocellular carcinoma; system pharmacology.
Copyright © 2020 Luo, Song, Wang, Huang, Liu, Wang, Hong and Yuan.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

