ACE2 Expression in the Cat and the Tiger Gastrointestinal Tracts
- PMID: 32903561
- PMCID: PMC7438561
- DOI: 10.3389/fvets.2020.00514
ACE2 Expression in the Cat and the Tiger Gastrointestinal Tracts
Abstract
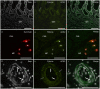
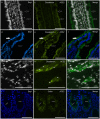
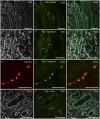
Angiotensin-converting enzyme 2 (ACE2) has been identified as the functional receptor for Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2). It has been identified in the human gastrointestinal tract (GIT), and SARS-CoV-2 has been isolated in human and animal fecal samples. The aim of the present study was to investigate the expression of ACE2 in the gastrointestinal tract of domestic (cat) and wild (tiger) felines. Samples of the pylorus, duodenum, and distal colon were collected from six cats and one tiger. The tissues were processed for immunofluorescence assay with an anti-human ACE2 antibody. Angiotensin-converting enzyme 2 was widely expressed in the gastrointestinal mucosa of the cats and the tiger. In both the species, ACE2-immunoreactivity (ACE2-IR) was expressed by the mucosal epithelial cells of the GIT and by the enteric neurons. In the cats, ACE2-IR was also expressed by the smooth muscle cells of the blood vessels and the tunica muscularis. The expression of the ACE2 receptor in enteric neurons may support the potential neurotropic properties of SARS-CoV-2. Although the evidence of ACE2-IR in the feline GIT does not necessarily indicate the possibility of viral replication and SARS-CoV-2 spread with stool, the findings in the present study could serve as an anatomical basis for additional studies considering the risk of the SARS-CoV-2 fecal-oral transmission between cats/felids, and between cats/felids and humans.
Keywords: COVID-19; SARS-CoV-2; angiotensin-converting enzyme 2; feline; immunohistochemistry.
Copyright © 2020 Chiocchetti, Galiazzo, Fracassi, Giancola and Pietra.
Figures
Similar articles
-
SARS-CoV-2 Clinical Outcome in Domestic and Wild Cats: A Systematic Review.Animals (Basel). 2021 Jul 9;11(7):2056. doi: 10.3390/ani11072056. Animals (Basel). 2021. PMID: 34359182 Free PMC article. Review.
-
Differential susceptibility of SARS-CoV-2 in animals: Evidence of ACE2 host receptor distribution in companion animals, livestock and wildlife by immunohistochemical characterisation.Transbound Emerg Dis. 2022 Jul;69(4):2275-2286. doi: 10.1111/tbed.14232. Epub 2021 Jul 26. Transbound Emerg Dis. 2022. PMID: 34245662 Free PMC article.
-
The Upper Respiratory Tract of Felids Is Highly Susceptible to SARS-CoV-2 Infection.Int J Mol Sci. 2021 Sep 30;22(19):10636. doi: 10.3390/ijms221910636. Int J Mol Sci. 2021. PMID: 34638978 Free PMC article.
-
ACE2 and Furin Expressions in Oral Epithelial Cells Possibly Facilitate COVID-19 Infection via Respiratory and Fecal-Oral Routes.Front Med (Lausanne). 2020 Dec 10;7:580796. doi: 10.3389/fmed.2020.580796. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33363183 Free PMC article.
-
COVID-19 and Neurological Manifestations.Brain Sci. 2023 Jul 29;13(8):1137. doi: 10.3390/brainsci13081137. Brain Sci. 2023. PMID: 37626493 Free PMC article. Review.
Cited by
-
SARS-CoV-2 Clinical Outcome in Domestic and Wild Cats: A Systematic Review.Animals (Basel). 2021 Jul 9;11(7):2056. doi: 10.3390/ani11072056. Animals (Basel). 2021. PMID: 34359182 Free PMC article. Review.
-
Detection and Genome Sequencing of SARS-CoV-2 in a Domestic Cat with Respiratory Signs in Switzerland.Viruses. 2021 Mar 17;13(3):496. doi: 10.3390/v13030496. Viruses. 2021. PMID: 33802899 Free PMC article.
-
Animal Transmission of SARS-CoV-2 and the Welfare of Animals during the COVID-19 Pandemic.Animals (Basel). 2021 Jul 8;11(7):2044. doi: 10.3390/ani11072044. Animals (Basel). 2021. PMID: 34359172 Free PMC article. Review.
-
COVID-19: pathogenesis, advances in treatment and vaccine development and environmental impact-an updated review.Environ Sci Pollut Res Int. 2021 May;28(18):22241-22264. doi: 10.1007/s11356-021-13018-1. Epub 2021 Mar 18. Environ Sci Pollut Res Int. 2021. PMID: 33733422 Free PMC article. Review.
-
Severe acute respiratory syndrome coronavirus 2 in cats: a systematic review.Braz J Vet Med. 2021 Jun 1;43:e000421. doi: 10.29374/2527-2179.bjvm000421. eCollection 2021. Braz J Vet Med. 2021. PMID: 35749089 Free PMC article.
References
-
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. (2020) 181:271–80. 10.1101/2020.01.31.929042 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

