Neonatal Tactile Stimulation Alters Behaviors in Heterozygous Serotonin Transporter Male Rats: Role of the Amygdala
- PMID: 32903627
- PMCID: PMC7438747
- DOI: 10.3389/fnbeh.2020.00142
Neonatal Tactile Stimulation Alters Behaviors in Heterozygous Serotonin Transporter Male Rats: Role of the Amygdala
Abstract
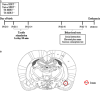
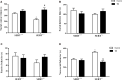
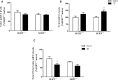
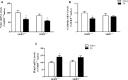
The serotonin transporter (SERT) gene, especially the short allele of the human serotonin transporter linked polymorphic region (5-HTTLPR), has been associated with the development of stress-related neuropsychiatric disorders. In line, exposure to early life stress in SERT knockout animals contributes to anxiety- and depression-like behavior. However, there is a lack of investigation of how early-life exposure to beneficial stimuli, such as tactile stimulation (TS), affects later life behavior in these animals. In this study, we investigated the effect of TS on social, anxiety, and anhedonic behavior in heterozygous SERT knockouts rats and wild-type controls and its impact on gene expression in the basolateral amygdala. Heterozygous SERT+/- rats were submitted to TS during postnatal days 8-14, for 10 min per day. In adulthood, rats were assessed for social and affective behavior. Besides, brain-derived neurotrophic factor (Bdnf) gene expression and its isoforms, components of glutamatergic and GABAergic systems as well as glucocorticoid-responsive genes were measured in the basolateral amygdala. We found that exposure to neonatal TS improved social and affective behavior in SERT+/- animals compared to naïve SERT+/- animals and was normalized to the level of naïve SERT+/+ animals. At the molecular level, we observed that TS per se affected Bdnf, the glucocorticoid-responsive genes Nr4a1, Gadd45β, the co-chaperone Fkbp5 as well as glutamatergic and GABAergic gene expression markers including the enzyme Gad67, the vesicular GABA transporter, and the vesicular glutamate transporter genes. Our results suggest that exposure of SERT+/- rats to neonatal TS can normalize their phenotype in adulthood and that TS per se alters the expression of plasticity and stress-related genes in the basolateral amygdala. These findings demonstrate the potential effect of a supportive stimulus in SERT rodents, which are more susceptible to develop psychiatric disorders.
Keywords: Bdnf; amygdala; anxiety; neonatal period; serotonin transporter knockout; tactile stimulation.
Copyright © 2020 Roversi, Buizza, Brivio, Calabrese, Verheij, Antoniazzi, Burger, Riva and Homberg.
Figures




Similar articles
-
Maternal separation induces anhedonia in female heterozygous serotonin transporter knockout rats.Behav Brain Res. 2019 Jan 1;356:204-207. doi: 10.1016/j.bbr.2018.08.031. Epub 2018 Aug 31. Behav Brain Res. 2019. PMID: 30176268
-
Neonatal tactile stimulation decreases depression-like and anxiety-like behaviors and potentiates sertraline action in young rats.Int J Dev Neurosci. 2015 Dec;47(Pt B):192-7. doi: 10.1016/j.ijdevneu.2015.09.010. Epub 2015 Oct 9. Int J Dev Neurosci. 2015. PMID: 26449401
-
Developmental influence of the serotonin transporter on the expression of npas4 and GABAergic markers: modulation by antidepressant treatment.Neuropsychopharmacology. 2012 Feb;37(3):746-58. doi: 10.1038/npp.2011.252. Epub 2011 Oct 19. Neuropsychopharmacology. 2012. PMID: 22012473 Free PMC article.
-
The Serotonin Transporter and Early Life Stress: Translational Perspectives.Front Cell Neurosci. 2017 Apr 26;11:117. doi: 10.3389/fncel.2017.00117. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28491024 Free PMC article. Review.
-
Experimental gene interaction studies with SERT mutant mice as models for human polygenic and epistatic traits and disorders.Genes Brain Behav. 2003 Dec;2(6):350-64. doi: 10.1046/j.1601-1848.2003.00049.x. Genes Brain Behav. 2003. PMID: 14653307 Review.
Cited by
-
Tactile Stimulation in Adult Rats Modulates Dopaminergic Molecular Parameters in the Nucleus accumbens Preventing Amphetamine Relapse.Mol Neurobiol. 2022 Sep;59(9):5564-5573. doi: 10.1007/s12035-022-02927-y. Epub 2022 Jun 22. Mol Neurobiol. 2022. PMID: 35732868 Free PMC article.
-
Early positive tactile stimulation reverses the increase of anxiety and decrease of sociality induced by early chronic mechanical pain in mandarin voles.Integr Zool. 2025 Jul;20(4):712-727. doi: 10.1111/1749-4877.12907. Epub 2024 Sep 23. Integr Zool. 2025. PMID: 39308260 Free PMC article.
-
A neural model of vulnerability and resilience to stress-related disorders linked to differential susceptibility.Mol Psychiatry. 2022 Jan;27(1):514-524. doi: 10.1038/s41380-021-01047-8. Epub 2021 Mar 1. Mol Psychiatry. 2022. PMID: 33649455 Review.
-
Enrichment Environment Positively Influences Depression- and Anxiety-Like Behavior in Serotonin Transporter Knockout Rats through the Modulation of Neuroplasticity, Spine, and GABAergic Markers.Genes (Basel). 2020 Oct 23;11(11):1248. doi: 10.3390/genes11111248. Genes (Basel). 2020. PMID: 33114023 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

