Hybrid Agent-Based Modeling of Aspergillus fumigatus Infection to Quantitatively Investigate the Role of Pores of Kohn in Human Alveoli
- PMID: 32903715
- PMCID: PMC7438790
- DOI: 10.3389/fmicb.2020.01951
Hybrid Agent-Based Modeling of Aspergillus fumigatus Infection to Quantitatively Investigate the Role of Pores of Kohn in Human Alveoli
Abstract
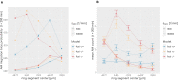
The healthy state of an organism is constantly threatened by external cues. Due to the daily inhalation of hundreds of particles and pathogens, the immune system needs to constantly accomplish the task of pathogen clearance in order to maintain this healthy state. However, infection dynamics are highly influenced by the peculiar anatomy of the human lung. Lung alveoli that are packed in alveolar sacs are interconnected by so called Pores of Kohn. Mainly due to the lack of in vivo methods, the role of Pores of Kohn in the mammalian lung is still under debate and partly contradicting hypotheses remain to be investigated. Although it was shown by electron microscopy that Pores of Kohn may serve as passageways for immune cells, their impact on the infection dynamics in the lung is still unknown under in vivo conditions. In the present study, we apply a hybrid agent-based infection model to quantitatively compare three different scenarios and discuss the importance of Pores of Kohn during infections of Aspergillus fumigatus. A. fumigatus is an airborne opportunistic fungus with rising incidences causing severe infections in immunocompromised patients that are associated with high mortality rates. Our hybrid agent-based model incorporates immune cell dynamics of alveolar macrophages - the resident phagocytes in the lung - as well as molecular dynamics of diffusing chemokines that attract alveolar macrophages to the site of infection. Consequently, this model allows a quantitative comparison of three different scenarios and to study the importance of Pores of Kohn. This enables us to demonstrate how passaging of alveolar macrophages and chemokine diffusion affect A. fumigatus infection dynamics. We show that Pores of Kohn alter important infection clearance mechanisms, such as the spatial distribution of macrophages and the effect of chemokine signaling. However, despite these differences, a lack of passageways for alveolar macrophages does impede infection clearance only to a minor extend. Furthermore, we quantify the importance of recruited macrophages in comparison to resident macrophages.
Keywords: Aspergillus fumigatus lung infection; Pores of Kohn; human model; hybrid agent-based computer simulations; virtual infection modeling.
Copyright © 2020 Blickensdorf, Timme and Figge.
Figures


References
-
- Adams W. E., Livingstone H. M. (1931). Obstructive pulmonary atelectasis. Arch. Surg. 23:500 10.1001/archsurg.1931.01160090145005 - DOI
-
- Barrett K., Barman S., Boitano S., Brooks H. (2012). Ganong’s Review of Medical Physiology, 24th Edn New York, NY: McGraw-Hill.
LinkOut - more resources
Full Text Sources

