Reg-1α Promotes Differentiation of Cortical Progenitors via Its N-Terminal Active Domain
- PMID: 32903776
- PMCID: PMC7443566
- DOI: 10.3389/fcell.2020.00681
Reg-1α Promotes Differentiation of Cortical Progenitors via Its N-Terminal Active Domain
Abstract
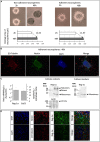
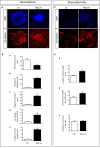
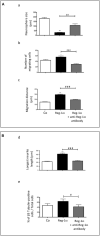
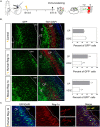
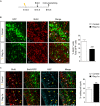
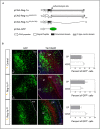
Reg-1α belongs to the Reg family of small, secreted proteins expressed in both pancreas and nervous system. Reg-1α is composed of two domains, an insoluble C-type lectin domain and a short soluble N-terminal peptide, which is released from the molecule upon proteolytic N-terminal processing, although the biological significance of this proteolysis remains unclear. We have previously shown that binding of Reg-1α to its receptor Extl3 stimulates axonal outgrowth. Reg-1α and Extl3 genes are expressed in the developing cortex but their expression decreases in adulthood, pointing to a possible function of this signaling system at the early developmental stages. Here, we demonstrate that recombinant Reg-1α increases migration and differentiation of cultured embryonic rat telencephalic progenitors via the activation of GSK-3β activity. In vivo overexpression of Reg-1α by in utero electroporation, has a similar effect, favoring premature differentiation of cortical progenitors. Notably, the N-terminal soluble domain, but not the C-type lectin domain, is largely responsible for Reg-1α effects on cortical neuronal differentiation. We thus conclude that Reg-1α via its proteolytically generated N-terminal domain is required for basic development processes.
Keywords: Extl3; GSK-3β; Reg-1α; axon elongation; differentiation; neural progenitors.
Copyright © 2020 Varilh, Acquatella-Tran Van Ba, Silhol, Nieto-Lopez, Moussaed, Lebart, Bovolenta, Verdier, Rossel, Marcilhac and Trousse.
Figures



References
-
- Acquatella-Tran Van Ba I., Marchal S., François F., Silhol M., Lleres C., Michel B., et al. (2012). Regenerating islet-derived 1α (Reg-1α) protein is new neuronal secreted factor that stimulates neurite outgrowth via exostosin Tumor-like 3 (EXTL3) receptor. J. Biol. Chem. 287 4726–4739. 10.1074/jbc.m111.260349 - DOI - PMC - PubMed
-
- Cerini C., Peyrot V., Garnier C., Duplan L., Veesler S., Caer J.-P. L., et al. (1999). Biophysical characterization of lithostathine evidences for A polymeric structure at physiological Ph and A proteolysis mechanism leading to the formation of fibrils. J. Biol. Chem. 274 22266–22274. 10.1074/jbc.274.32.22266 - DOI - PubMed

