The Efficacy and Safety of Cytarabine on Newly Diagnosed Primary Central Nervous System Lymphoma: A Systematic Review and Meta-Analysis
- PMID: 32903796
- PMCID: PMC7438862
- DOI: 10.3389/fonc.2020.01213
The Efficacy and Safety of Cytarabine on Newly Diagnosed Primary Central Nervous System Lymphoma: A Systematic Review and Meta-Analysis
Abstract
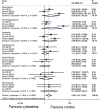
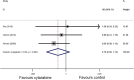
Background: The role of cytarabine on newly diagnosed primary central nervous system lymphoma (PCNSL) remains controversial. The present study mainly aimed to assess the efficacy and safety of cytarabine in the induction treatment of PCNSL. Methods: We systematically searched PubMed, Embase, and the Cochrane library for randomized controlled trials comparing treatment of PCNSL patients with or without cytarabine. A meta-analysis was conducted to compare the odds ratios (ORs) with corresponding 95% confidence intervals (95% CI) for complete remission (CR) rate, overall response rate (ORR), grade 3-4 toxic effects, hazard ratios (HRs) with 95% CIs for progression-free survival (PFS), and overall survival (OS) using Stata 12.0. Results: In total, three randomized clinical trials were analyzed in this study. The result of our statistical analysis demonstrated that the application of cytarabine was closely correlated with a higher CR (OR: 2.27, 95% CI: 1.29-3.99, P < 0.01) and ORR (OR: 2.11, 95% CI: 1.14-3.93, P = 0.02). No significant difference was found in OS (HR: 0.75, 95% CI: 0.50-1.13, P = 0.17), but PFS had been improved (HR: 0.66, 95% CI: 0.45-0.97, P = 0.04) when cytarabine was added to the treatment regimen. The grade 3-4 side effect rate of the cytarabine group was higher (overall OR: 2.95, 95% CI: 1.37-6.34, P < 0.01) than that of the cytarabine-free group. Conclusions: This meta-analysis verifies that adding cytarabine to the therapeutic regimen is helpful for newly diagnosed PCNSL patients in terms of CR, ORR, and PFS. Moreover, it should be noted that the grade 3-4 toxic effects, especially hematological toxicity, are higher in the cytarabine group than in the cytarabine-free group. The results indicate that cytarabine plays an important role in the induction therapy of PCNSL. Large-sample and high-quality RCTs should be conducted to verify our results and confirm the effects of cytarabine on newly diagnosed PCNSL.
Keywords: brain tumor; chemotherapy; cytarabine; meta-analysis; primary central nervous system lymphoma.
Copyright © 2020 Zheng, Yang, Chen, Wu and Li.
Figures



References
-
- Schorb E, Finke J, Ferreri AJM, Ihorst G, Mikesch K, Kasenda B, et al. . High-dose chemotherapy and autologous stem cell transplant compared with conventional chemotherapy for consolidation in newly diagnosed primary CNS lymphoma-a randomized phase III trial (MATRix). BMC Cancer. (2016) 16:282. 10.1186/s12885-016-2311-4 - DOI - PMC - PubMed
-
- Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, et al. . High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. (2009) 374:1512–20. 10.1016/S0140-6736(09)61416-1 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

