Eye Drops of Metformin Prevents Fibrosis After Glaucoma Filtration Surgery in Rats via Activating AMPK/Nrf2 Signaling Pathway
- PMID: 32903813
- PMCID: PMC7438907
- DOI: 10.3389/fphar.2020.01038
Eye Drops of Metformin Prevents Fibrosis After Glaucoma Filtration Surgery in Rats via Activating AMPK/Nrf2 Signaling Pathway
Abstract
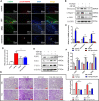
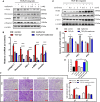
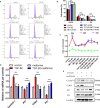
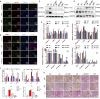
Metformin has effective therapeutic effects in anti-tumor and anti-fibrotic diseases. However, how the antifibrotic effect of metformin in the eye and how it is transferred are still unclear. Here, the eye drop of metformin treatment was studied in Sprague-Dawley (SD) rats of glaucoma filtrating surgery (GFS). Rats were administered randomly bilateral drops: control group (without surgery), GFS group, metformin group or mitomycin C (MMC) group (sponge application intraoperatively, 0.02%). Bleb features and intraocular pressure (IOP) were assessed for postoperative week 4. Metformin effectively inhibited fibrosis and improved the surgical outcomes of GFS. In vitro, we found that the degree of oxidative stress and fibrosis in metformin pretreated-Human Conjunctival Fibroblasts (HConFs) were reduced; the pro-fibrotic response of HConFs were decreased by inducing macrophagic polarity changes. Besides, the inhibition of nuclear factor erythroid 2-related factor 2 (Nrf2)/AMP-activated protein kinase (AMPK) and the competition of organic cation transporters (OCTs) effectively reduced the anti-fibrotic capability of metformin. Together, this experiment indicates that metformin enters into HConFs cell with OCTs, which can protect against filtrating blebs scar formation in SD rats of GFS via activating AMPK/Nrf2 axis and the downregulation of profibrogenic and inflammatory biomarkers.
Keywords: AMPK/Nrf2; fibrosis; glaucoma filtration surgery (GFS); macrophages; metformin; organic cation transporters (OCTs); oxidative stress.
Copyright © 2020 Li, Leng, Jiang, Wang, Luo, Zhang, Chen, Wang, Wang, Yue, Shen, Zhou, Shi and Xie.
Figures



Similar articles
-
Targeting CCL5 Attenuates Fibrosis via Activation of PI3k/Akt Signaling Axis After Glaucoma Filtration Surgery.Curr Eye Res. 2025 Apr;50(4):394-404. doi: 10.1080/02713683.2024.2432399. Epub 2024 Dec 2. Curr Eye Res. 2025. PMID: 39618346
-
The TβR II-targeted aptamer S58 prevents fibrosis after glaucoma filtration surgery.Aging (Albany NY). 2020 May 23;12(10):8837-8857. doi: 10.18632/aging.102997. Epub 2020 May 23. Aging (Albany NY). 2020. PMID: 32452828 Free PMC article.
-
α5β1-Integrin inhibitor (CLT-28643) effective in rabbit trabeculectomy model.Acta Ophthalmol. 2017 Feb;95(1):e1-e9. doi: 10.1111/aos.13215. Epub 2016 Aug 31. Acta Ophthalmol. 2017. PMID: 27576860
-
[A challenge to primary open-angle glaucoma including normal-pressure. Clinical problems and their scientific solution].Nippon Ganka Gakkai Zasshi. 2012 Mar;116(3):233-67; discussion 268. Nippon Ganka Gakkai Zasshi. 2012. PMID: 22568103 Review. Japanese.
-
Metformin and Glaucoma-Review of Anti-Fibrotic Processes and Bioenergetics.Cells. 2021 Aug 19;10(8):2131. doi: 10.3390/cells10082131. Cells. 2021. PMID: 34440899 Free PMC article. Review.
Cited by
-
Genome-wide RNA sequencing of ocular fibroblasts from glaucomatous and normal eyes: Implications for glaucoma management.PLoS One. 2024 Jul 11;19(7):e0307227. doi: 10.1371/journal.pone.0307227. eCollection 2024. PLoS One. 2024. PMID: 38990974 Free PMC article.
-
Autophagy in glaucoma pathogenesis: Therapeutic potential and future perspectives.Front Cell Dev Biol. 2022 Dec 14;10:1068213. doi: 10.3389/fcell.2022.1068213. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36589756 Free PMC article. Review.
-
Metformin Alleviates Fibrosis of Trabecular Meshwork Cells Induced by TGFβ2 Through the Activation of Autophagy.Dose Response. 2025 May 20;23(2):15593258251341598. doi: 10.1177/15593258251341598. eCollection 2025 Apr-Jun. Dose Response. 2025. PMID: 40401245 Free PMC article.
-
Rutin ameliorates imiquimod-induced psoriasis-like skin lesions by inhibiting oxidative stress injury and the inflammatory response in mice via the Keap1/Nrf2 signaling pathway.Sci Rep. 2025 Jul 1;15(1):20712. doi: 10.1038/s41598-025-08451-y. Sci Rep. 2025. PMID: 40596643 Free PMC article.
-
Recent developments of neuroprotective agents for degenerative retinal disorders.Neural Regen Res. 2022 Sep;17(9):1919-1928. doi: 10.4103/1673-5374.335140. Neural Regen Res. 2022. PMID: 35142668 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

