Saliva as a Candidate for COVID-19 Diagnostic Testing: A Meta-Analysis
- PMID: 32903849
- PMCID: PMC7438940
- DOI: 10.3389/fmed.2020.00465
Saliva as a Candidate for COVID-19 Diagnostic Testing: A Meta-Analysis
Abstract
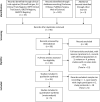
Background: COVID-19 is a serious and potentially deadly disease. Early diagnosis of infected individuals will play an important role in stopping its further escalation. The present gold standard for sampling is the nasopharyngeal swab method. However, several recent papers suggested that saliva-based testing is a promising alternative that could simplify and accelerate COVID-19 diagnosis. Objectives: Our aim was to conduct a meta-analysis on the reliability and consistency of SARS-CoV-2 viral RNA detection in saliva specimens. Methods: We have reported our meta-analysis according to the Cochrane Handbook. We searched the Cochrane Library, Embase, Pubmed, Scopus, Web of Science and clinical trial registries for eligible studies published between 1 January and 25 April 2020. The number of positive tests and the total number of tests conducted were collected as raw data. The proportion of positive tests in the pooled data were calculated by score confidence-interval estimation with the Freeman-Tukey transformation. Heterogeneity was assessed using the I 2 measure and the χ2-test. Results: The systematic search revealed 96 records after removal of duplicates. Twenty-six records were included for qualitative analysis and 5 records for quantitative synthesis. We found 91% (CI 80-99%) sensitivity for saliva tests and 98% (CI 89-100%) sensitivity for nasopharyngeal swab (NPS) tests in previously confirmed COVID-19 patients, with moderate heterogeneity among the studies. Additionally, we identified 18 registered, ongoing clinical trials of saliva-based tests for detection of the virus. Conclusion: Saliva tests offer a promising alternative to NPS for COVID-19 diagnosis. However, further diagnostic accuracy studies are needed to improve their specificity and sensitivity.
Keywords: COVID-19; SARS-CoV-2; coronavirus; diagnostic tests; meta-analysis; saliva; systematic review.
Copyright © 2020 Czumbel, Kiss, Farkas, Mandel, Hegyi, Nagy, Lohinai, Szakács, Hegyi, Steward and Varga.
Figures

References
-
- World Health O WHO Coronavirus Disease (COVID-19) Dashboard (2020). Retrieved from: https://covid19.who.int (accessed May 05, 2020).
-
- World Health O Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance. Geneva (2020). Retrieved from: https://www.who.int/publications-detail/laboratory-testing-for-2019-nove... (accessed March 19, 2020).
Publication types
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

