Prevalence, Serotype, Antibiotic Susceptibility, and Genotype of Salmonella in Eggs From Poultry Farms and Marketplaces in Yangling, Shaanxi Province, China
- PMID: 32903897
- PMCID: PMC7438954
- DOI: 10.3389/fmicb.2020.01482
Prevalence, Serotype, Antibiotic Susceptibility, and Genotype of Salmonella in Eggs From Poultry Farms and Marketplaces in Yangling, Shaanxi Province, China
Abstract
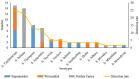
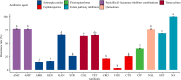
Poultry products such as eggs provide essential nutrients to the human body and thus play vital roles in the human food network. Salmonella is one of the most notorious foodborne pathogens and has been found to be prevalent in eggs. To better understand the characteristics of Salmonella in eggs, we investigated the prevalence of Salmonella spp. in 814 fresh eggs collected from poultry farms and retail marketplaces in Yangling, Shaanxi Province, China. The serotype, genotype, and antibiotic susceptibilities of 61 Salmonella isolates recovered from the eggs were analyzed. The average detection rate of Salmonella-positive eggs was 5.6%, with 6.6% of the eggs collected from poultry farms and 5.1% from marketplaces. Thirteen serotypes were identified from the 61 isolates, among which Salmonella Typhimurium (24.5%) and Salmonella Indiana (22.9%) were the most prevalent serotypes. Other dominant serotypes included Salmonella Thompson (13.1%) and Salmonella Enteritidis (11.4%), with the remaining nine serotypes detected at low rates (1.6-4.9%). All the Salmonella isolates tested were resistant to sulfisoxazole (100.0%). The majority (77.1%) of the isolates were resistant to nalidixic acid, amoxicillin-clavulanate, and ampicillin, while nearly two-thirds (63.9-68.9%) were resistant to trimethoprim-sulfamethoxazole, kanamycin, tetracyclines, and chloramphenicol. The rate of resistance to ciprofloxacin was 40.1%; the resistance rates to streptomycin, ceftiofur, and ceftriaxone ranged from 21.3 to 26.2%; and those to gentamicin, amikacin, and cefoxitin were relatively low (3.3-16.4%). Forty-nine (80.3%) Salmonella isolates exhibited resistance to multiple antibiotics, 20 (32.8%) of which were resistant to at least 10 antibiotics. Subtyping by pulse-field gel electrophoresis revealed a close genetic relatedness of Salmonella isolates from poultry farms, in striking contrast to the high diversity of the isolates obtained from marketplaces. Isolates of the same serotype always shared identical genotype and antibiotic resistance profiles, even the ones that were recovered from eggs sampled at different locations and times. These findings indicate that diverse Salmonella spp. with high rates of multidrug resistance are prevalent in fresh eggs in the study area. More attention should be paid to egg production, transportation, and storage to prevent foodborne outbreaks caused by Salmonella.
Keywords: Salmonella; antibiotic resistance; eggs; genotype; serotype.
Copyright © 2020 Li, Li, Zheng, Wang, Sheng, Shi, Shi, Niu and Yang.
Figures



Similar articles
-
Prevalence and characteristics of Salmonella isolates recovered from retail raw chickens in Shaanxi Province, China.Poult Sci. 2020 Nov;99(11):6031-6044. doi: 10.1016/j.psj.2020.07.038. Epub 2020 Aug 12. Poult Sci. 2020. PMID: 33142522 Free PMC article.
-
Diversity of Serotype, Genotype, and Antibiotic Susceptibility of Salmonella Prevalent in Pickled Ready-to-Eat Meat.Front Microbiol. 2019 Nov 12;10:2577. doi: 10.3389/fmicb.2019.02577. eCollection 2019. Front Microbiol. 2019. PMID: 31781073 Free PMC article.
-
Comparative Study on Antibiotic Resistance and DNA Profiles of Salmonella enterica Serovar Typhimurium Isolated from Humans, Retail Foods, and the Environment in Shanghai, China.Foodborne Pathog Dis. 2018 Aug;15(8):481-488. doi: 10.1089/fpd.2017.2414. Epub 2018 May 9. Foodborne Pathog Dis. 2018. PMID: 29741928
-
Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview.Vet World. 2020 Oct;13(10):2070-2084. doi: 10.14202/vetworld.2020.2070-2084. Epub 2020 Oct 3. Vet World. 2020. PMID: 33281339 Free PMC article. Review.
-
Global prevalence and antibiotic resistance profiles of bacterial pathogens in table eggs: A systematic review and meta-analysis.Vet World. 2025 Apr;18(4):939-954. doi: 10.14202/vetworld.2025.939-954. Epub 2025 Apr 23. Vet World. 2025. PMID: 40453930 Free PMC article. Review.
Cited by
-
Occurrence and characterization of Salmonella isolates from commercial eggs in Phayao Province, Thailand.Vet World. 2025 Mar;18(3):705-714. doi: 10.14202/vetworld.2025.705-714. Epub 2025 Mar 23. Vet World. 2025. PMID: 40342750 Free PMC article.
-
Genomic Features and Phylogenetic Analysis of Antimicrobial-Resistant Salmonella Mbandaka ST413 Strains.Microorganisms. 2024 Feb 1;12(2):312. doi: 10.3390/microorganisms12020312. Microorganisms. 2024. PMID: 38399716 Free PMC article.
-
Resistance profiles, virulence and antimicrobial resistance genes of XDR S. Enteritidis and S. Typhimurium.AMB Express. 2023 Oct 10;13(1):110. doi: 10.1186/s13568-023-01615-x. AMB Express. 2023. PMID: 37817026 Free PMC article.
-
A pan-genome perspective on the evolutionary dynamics of polyphyly, virulence, and antibiotic resistance in Salmonella enterica serovar Mbandaka highlights emerging threats to public health and food safety posed by cloud gene families.Curr Res Food Sci. 2024 Dec 13;10:100957. doi: 10.1016/j.crfs.2024.100957. eCollection 2025. Curr Res Food Sci. 2024. PMID: 39802648 Free PMC article.
-
Multiplex PCR-based genotyping of Salmonella Enteritidis and Salmonella Typhimurium from food sources and assessment of their antimicrobial resistance profiles in Egypt.Mol Biol Rep. 2024 Jul 13;51(1):794. doi: 10.1007/s11033-024-09704-1. Mol Biol Rep. 2024. PMID: 39001999
References
-
- Al S., Hizlisoy H., Ertas Onmaz N., Yildirim Y., Gonulalan Z. (2015). Occurrence and antimicrobial resistance of Salmonella enterica subsp. enterica serovars Typhimurium, Enteritidis, and Typhi isolated from chicken eggs and poultry products. Turk. J. Vet. Anim. Sci. 40 737–743. 10.3906/vet-1601-17 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

