Efficacy of Sub-Tenon Micro-Perfusion of Cyclophosphamide in Rabbits with Severe Ocular Inflammation
- PMID: 32903954
- PMCID: PMC7445515
- DOI: 10.2147/DDDT.S250541
Efficacy of Sub-Tenon Micro-Perfusion of Cyclophosphamide in Rabbits with Severe Ocular Inflammation
Abstract
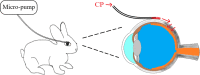
Purpose: To explore the feasibility of cyclophosphamide (CP) via a sub-Tenon micro-perfusion system (SMS) in rabbits, and assess its therapeutic efficacy in severe ocular inflammation.
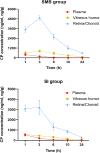
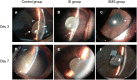
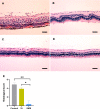
Materials and methods: Distribution and pharmacokinetics of CP were evaluated in vivo, and the concentrations of CP in plasma, vitreous humor, and retina/choroid were quantitated by ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) at different time points. After induction of severe experimental uveitis, rabbits were divided into three groups (n=8 in each): the SMS group, subconjunctival injection (SI) group, and control group. Clinical inflammatory score was assessed in rabbits. Electroretinography and histopathology were performed on post-treatment day 8. Statistical analyses were performed using Mann-Whitney and Kruskal-Wallis tests. P-value less than 0.05 was considered significant.
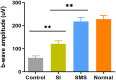
Results: The concentrations of CP in vitreous humor and retina/choroid in the SMS group were significantly higher than that of the SI group at 3, 6, 10, and 24 hours (P<0.01), while plasmatic CP concentrations were comparable at all time points in the SMS group and SI group (P>0.05). The SMS group showed significantly less inflammation compared to the control group and SI group. Furthermore, the restoration of retinal structure and function were more obvious in the SMS group compared with conventional SI application.
Conclusion: Sub-Tenon micro-perfusion of CP exhibited satisfied therapeutic efficacy in rabbits with severe ocular inflammation and may provide a promising alternative for controlling ocular inflammatory disease and immune-mediated ocular diseases.
Keywords: cyclophosphamide; ocular inflammation; rabbit; sub-Tenon drug delivery; treatment.
© 2020 Zhao et al.
Conflict of interest statement
All authors declare no conflicts of interest in this work.
Figures

Similar articles
-
Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model.J Control Release. 2015 Apr 10;203:109-17. doi: 10.1016/j.jconrel.2015.02.021. Epub 2015 Feb 17. J Control Release. 2015. PMID: 25700623
-
Sub-Tenon Sustained Controllable Delivery of Dexamethasone for Treating Severe Acute Experimental Uveitis.Ocul Immunol Inflamm. 2020 Aug 17;28(6):984-993. doi: 10.1080/09273948.2019.1643027. Epub 2019 Aug 20. Ocul Immunol Inflamm. 2020. PMID: 31429619
-
Scleral plug of biodegradable polymers containing tacrolimus (FK506) for experimental uveitis.Invest Ophthalmol Vis Sci. 2003 Nov;44(11):4845-52. doi: 10.1167/iovs.02-1228. Invest Ophthalmol Vis Sci. 2003. PMID: 14578407
-
Distribution of topical ocular nepafenac and its active metabolite amfenac to the posterior segment of the eye.Exp Eye Res. 2016 Apr;145:58-67. doi: 10.1016/j.exer.2015.10.009. Epub 2015 Oct 22. Exp Eye Res. 2016. PMID: 26474497
-
A Low Concentration of Tacrolimus/Semifluorinated Alkane (SFA) Eyedrop Suppresses Intraocular Inflammation in Experimental Models of Uveitis.Curr Mol Med. 2017;17(3):211-220. doi: 10.2174/1566524017666170807144009. Curr Mol Med. 2017. PMID: 28782485 Free PMC article.
Cited by
-
A Review of Ocular Drug Delivery Platforms and Drugs for Infectious and Noninfectious Uveitis: The Past, Present, and Future.Pharmaceutics. 2021 Aug 8;13(8):1224. doi: 10.3390/pharmaceutics13081224. Pharmaceutics. 2021. PMID: 34452185 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

