Analysis of Funding Source and Spin in the Reporting of Studies of Intravitreal Corticosteroid Therapy for Diabetic Macular Edema: A Systematic Review
- PMID: 32903959
- PMCID: PMC7445525
- DOI: 10.2147/OPTH.S262085
Analysis of Funding Source and Spin in the Reporting of Studies of Intravitreal Corticosteroid Therapy for Diabetic Macular Edema: A Systematic Review
Abstract
Purpose: This systematic review examined the relationship between industry funding and the presence of spin in high-impact studies evaluating intravitreal corticosteroid therapy for diabetic macular edema.
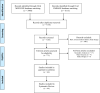
Methods: This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. MEDLINE and Embase were systematically searched from inception through July 16, 2018, for randomized controlled trials and meta-analyses investigating the treatment of patients with diabetic macular edema using intravitreal corticosteroid therapy. Only studies published in English journals with an impact factor greater than 2 as per the Clarivate Analytics 2017 Journal Citation Report were included. The authors independently assessed study quality, funding source and the presence of reporting bias using a standardized datasheet.
Results: Title and abstract screening were completed on 7158 unique hits and full-text review yielded 44 included studies. Overall, there was correspondence between the wording of abstract conclusions and study results in 41/44 (93%) articles. Correspondence between abstract conclusions and significance of main outcome was present in 14/14 (100%) industry-funded and 27/30 (90%) nonindustry-funded studies. The odds ratio of industry funding being associated with noncorrespondence was 0.27 (95% CI: 0.01-5.61, p=0.54). The most common reason for noncorrespondence was the failure to mention rates of steroid-related intraocular pressure elevation.
Conclusion: The results of this systematic review indicate that biased abstract outcome reporting is rare in published randomized controlled trials and meta-analyses of intravitreal corticosteroid therapy for diabetic macular edema. Biased reporting was not associated with the presence of industry funding or a conflict of interest.
Keywords: corticosteroids; diabetic retinopathy; intravitreal therapy; macular edema; systematic review.
© 2020 Nithianandan et al.
Conflict of interest statement
JS is a consultant for Alcon Laboratories, Alimera Science, Oxurion, Regeneron, and Thrombogenics. AEK is a consultant for Allergan, Alimera Sciences, Regeneron, and Valeant and receives grant funding from Bausch Health, Roche/Genentech and Second Sight. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Effect of funding source on reporting bias in studies of intravitreal anti-vascular endothelial growth factor therapy for retinal vein occlusion.Acta Ophthalmol. 2019 Mar;97(2):e296-e302. doi: 10.1111/aos.13917. Epub 2018 Sep 19. Acta Ophthalmol. 2019. PMID: 30232841
-
Effect of Funding Source on "Spin" in Studies of Ocriplasmin Therapy for Vitreomacular Traction and Macular Hole.Clin Ophthalmol. 2020 Jan 13;14:81-88. doi: 10.2147/OPTH.S233816. eCollection 2020. Clin Ophthalmol. 2020. PMID: 32021071 Free PMC article.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Discrepancy between results and abstract conclusions in industry- vs nonindustry-funded studies comparing topical prostaglandins.Am J Ophthalmol. 2009 Jan;147(1):33-38.e2. doi: 10.1016/j.ajo.2008.07.005. Epub 2008 Aug 30. Am J Ophthalmol. 2009. PMID: 18760766
-
Intravitreal Pharmacotherapies for Diabetic Macular Edema: A Report by the American Academy of Ophthalmology.Ophthalmology. 2022 Jan;129(1):88-99. doi: 10.1016/j.ophtha.2021.07.009. Epub 2021 Aug 23. Ophthalmology. 2022. PMID: 34446301 Review.
References
Publication types
LinkOut - more resources
Full Text Sources