Picosecond Laser-Induced Photothermal Skin Damage Evaluation by Computational Clinical Trial
- PMID: 32903975
- PMCID: PMC7447831
- DOI: 10.5978/islsm.20-OR-08
Picosecond Laser-Induced Photothermal Skin Damage Evaluation by Computational Clinical Trial
Abstract
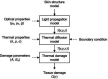
Background and objectives: Computational clinical trial (CCT) in the field of laser medicine promotes clinical application of novel laser devices, because this trial carried out based on numerical modeling of laser-tissue interactions and simulation of a series of treatment process. To confirm the feasibility of the computational clinical trial of skin treatment with a novel picosecond laser, this paper presents an evaluation method of the safety.
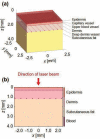
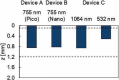
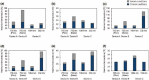
Study design/materials and methods: In this method, the light propagation and thermal diffusion process after ultrashort light pulse irradiation to a numerical skin model is calculated and the safety based on the photothermal damage is evaluated by computational modeling and simulation. As an example, the safety of a novel picosecond laser device was examined by comparing with several laser devices approved for clinical use.
Results: The ratio of the maximum thermal damage induced by picosecond laser irradiation was 1.2 × 10-2 % at the epidermis, while that caused by approved laser irradiation was 99 % at the capillary vessels. The numerical simulation demonstrated that less thermal damage was observed compared with the approved devices. The results show the safety simulated by photothermal damage calculation was consistent with the reported clinical trials.
Conclusions: This computational clinical trial shows the feasibility of applying computational clinical trials for the safety evaluation of novel medical laser devices. In contrast to preclinical and clinical tests, the proposed computational method offers regulatory science for appropriately and quickly predicting and evaluating the safety of a novel laser device.
Keywords: Medical device evaluation; computational clinical trial; photothermal damage; picosecond laser; regulatory science; safety evaluation.
2020, Japan Medical Laser Laboratory.
Figures



Similar articles
-
Incident Fluence Analysis for 755-nm Picosecond Laser Treatment of Pigmented Skin Lesions Based on Threshold Fluences for Melanosome Disruption.Lasers Surg Med. 2021 Oct;53(8):1096-1104. doi: 10.1002/lsm.23391. Epub 2021 Feb 19. Lasers Surg Med. 2021. PMID: 33604920 Free PMC article.
-
Guide mapping for effective superficial photothermal coagulation of the esophagus using computer simulations with ex vivo sheep model validation study.Lasers Surg Med. 2022 Oct;54(8):1116-1129. doi: 10.1002/lsm.23595. Epub 2022 Sep 1. Lasers Surg Med. 2022. PMID: 36047422
-
Treatment of pigmentary disorders in patients with skin of color with a novel 755 nm picosecond, Q-switched ruby, and Q-switched Nd:YAG nanosecond lasers: A retrospective photographic review.Lasers Surg Med. 2016 Feb;48(2):181-7. doi: 10.1002/lsm.22454. Lasers Surg Med. 2016. PMID: 26922302
-
A Review of Numerical Simulation and Analytical Modeling for Medical Devices Safety in MRI.Yearb Med Inform. 2016 Nov 10;(1):152-158. doi: 10.15265/IY-2016-016. Yearb Med Inform. 2016. PMID: 27830244 Free PMC article. Review.
-
Lasers in tattoo and pigmentation control: role of the PicoSure(®) laser system.Med Devices (Auckl). 2016 May 2;9:63-7. doi: 10.2147/MDER.S77993. eCollection 2016. Med Devices (Auckl). 2016. PMID: 27194919 Free PMC article. Review.
Cited by
-
Transient simulation of laser ablation based on Monte Carlo light transport with dynamic optical properties model.Sci Rep. 2023 Jul 24;13(1):11898. doi: 10.1038/s41598-023-39026-4. Sci Rep. 2023. PMID: 37488156 Free PMC article.
-
Ultralow radiant exposure of a short-pulsed laser to disrupt melanosomes with localized thermal damage through a turbid medium.Sci Rep. 2024 Aug 29;14(1):20112. doi: 10.1038/s41598-024-70807-7. Sci Rep. 2024. PMID: 39209990 Free PMC article.
-
The State-of-the-Art and Perspectives of Laser Ablation for Tumor Treatment.Cyborg Bionic Syst. 2024 Jan 5;5:0062. doi: 10.34133/cbsystems.0062. eCollection 2024. Cyborg Bionic Syst. 2024. PMID: 38188984 Free PMC article.
-
Incident Fluence Analysis for 755-nm Picosecond Laser Treatment of Pigmented Skin Lesions Based on Threshold Fluences for Melanosome Disruption.Lasers Surg Med. 2021 Oct;53(8):1096-1104. doi: 10.1002/lsm.23391. Epub 2021 Feb 19. Lasers Surg Med. 2021. PMID: 33604920 Free PMC article.
-
Spatial distribution of light-melanosome interaction dependent on irradiation fluence and spot size for short-pulsed laser skin treatment: A phantom study.Lasers Med Sci. 2025 May 30;40(1):251. doi: 10.1007/s10103-025-04430-x. Lasers Med Sci. 2025. PMID: 40445442 Free PMC article.
References
-
- Pinto F, Große-Büning S, Karsai S, Weiß C, Bäumler W, Hammes S, Felcht M, Raulin C: Neodymium-doped yttrium aluminium garnet (Nd:YAG) 1064-nm picosecond laser vs. Nd:YAG 1064-nm nanosecond laser in tattoo removal: a randomized controlled single-blind clinical trial. Br J Dermatol, 2017; 176(2):457-64. - PubMed
-
- Levin MK, Ng E, Bae YSC, Brauer JA, Geronemus RG: Treatment of pigmentary disorders in patients with skin of color with a novel 755 nm picosecond, Q-switched ruby, and Q-switched Nd:YAG nanosecond lasers: A retrospective photographic review. Lasers Surg Med, 2016; 48(2):181-87. - PubMed
-
- Lee MC, Lin YF, Hu S, Huang YL, Chang SL, Cheng CY, Chang CS: A split-face study: comparison of picosecond alexandrite laser and Q-switched Nd:YAG laser in the treatment of melasma in Asians. Lasers Med Sci, 2018; 33(8):1733-8. - PubMed
-
- Vachiramon V, Iamsumang W, Triyangkulsri K: Q-switched double frequency Nd:YAG 532-nm nanosecond laser vs. double frequency Nd:YAG 532-nm picosecond laser for the treatment of solar lentigines in Asians. Lasers Med Sci, 2018; 33(9):1941-7. - PubMed
-
- Brauer LA, Kazlouskaya V, Alabdulrazzaq H, Bae YS, Bernstein LJ, Anolik R, Heller PA, Geronemus RG: Use of a picosecond pulse duration laser with specialized optic for treatment of facial acne scarring. JAMA Dermatol, 2015; 151(32):278-84. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
