TiO2 Nanoparticles Caused DNA Damage in Lung and Extra-Pulmonary Organs Through ROS-Activated FOXO3a Signaling Pathway After Intratracheal Administration in Rats
- PMID: 32904047
- PMCID: PMC7449758
- DOI: 10.2147/IJN.S254969
TiO2 Nanoparticles Caused DNA Damage in Lung and Extra-Pulmonary Organs Through ROS-Activated FOXO3a Signaling Pathway After Intratracheal Administration in Rats
Abstract
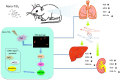
Introduction: Because of the increased production and application of manufactured Nano-TiO2 in the past several years, it is important to investigate its potential hazards. TiO2 is classified by IARC as a possible human carcinogen; however, the potential mechanism of carcinogenesis has not been studied clearly. The present study aimed to investigate the mechanism of DNA damage in rat lung and extra-pulmonary organs caused by TiO2nanoparticles.
Methods: In the present study, SD rats were exposed to Nano-TiO2 by intratracheal injection at a dose of 0, 0.2, or 1 g/kg body weight. The titanium levels in tissues were detected by ICP-MS. Western blot was used to detect the protein expression levels. The DNA damage and oxidative stress were detected by comet assay and ROS, MDA, SOD, and GSH-Px levels, respectively.
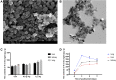
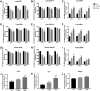
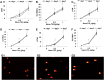
Results: The titanium levels of the 1 g/kg group on day-3 and day-7 were significantly increased in liver and kidney as well as significantly decreased in lung compared to day-1. ROS and MDA levels were statistically increased, whereas SOD and GSH-Px levels were statistically decreased in tissues of rats in dose-dependent manners after Nano-TiO2 treatment. PI3K, p-AKT/AKT, and p-FOXO3a/FOXO3a in lung, liver, and kidney activated in dose-dependent manners. The levels of DNA damage in liver, kidney, and lung in each Nano-TiO2 treatment group were significantly increased and could not recover within 7 days. GADD45α, ChK2, and XRCC1 in liver, kidney, and lung of rats exposed to Nano-TiO2 statistically increased, which triggered DNA repair.
Conclusion: This work demonstrated that Ti could deposit in lung and enter extra-pulmonary organs of rats and cause oxidative stress, then trigger DNA damage through activating the PI3K-AKT-FOXO3a pathway and then promoting GADD45α, ChK2, and XRCC1 to process the DNA repair.
Keywords: DNA damage; DNA repair; GADD45α/ChK2/XRCC1 signaling pathway; Nano-TiO2; PI3K/AKT/FOXO3a signaling pathway.
© 2020 Han et al.
Conflict of interest statement
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted. The authors report no conflicts of interest for this work.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

