Recent insights into natural product inhibitors of matrix metalloproteinases
- PMID: 32904148
- PMCID: PMC7451072
- DOI: 10.1039/c9md00165d
Recent insights into natural product inhibitors of matrix metalloproteinases
Abstract
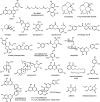
Members of the matrix metalloproteinase (MMP) family have biological functions that are central to human health and disease, and MMP inhibitors have been investigated for the treatment of cardiovascular disease, cancer and neurodegenerative disorders. The outcomes of initial clinical trials with the first generation of MMP inhibitors proved disappointing. However, our growing understanding of the complexities of the MMP function in disease, and an increased understanding of MMP protein architecture and control of activity now provide new opportunities and avenues to develop MMP-focused therapies. Natural products that affect MMP activities have been of strong interest as templates for drug discovery, and for their use as chemical tools to help delineate the roles of MMPs that still remain to be defined. Herein, we highlight the most recent discoveries of structurally diverse natural product inhibitors to these proteases.
This journal is © The Royal Society of Chemistry 2019.
Figures



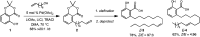
References
-
- Egeblad M., Werb Z. Nat. Rev. Cancer. 2002;2:161–174. - PubMed
-
- Parks W. C., Wilson C. L., Lopez-Boado Y. S. Nat. Rev. Immunol. 2004;4:617–629. - PubMed
-
- Milner J. M., Cawston T. E. Curr. Drug Targets: Inflammation Allergy. 2005;4:363–375. - PubMed
-
- Hu J., Van den Steen P. E., Sang Q. X., Opdenakker G. Nat. Rev. Drug Discovery. 2007;6:480–498. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

