Efficacy and Tolerability of a Fixed-Dose Combination of Rosuvastatin and Ezetimibe Compared with a Fixed-Dose Combination of Simvastatin and Ezetimibe in Brazilian Patients with Primary Hypercholesterolemia or Mixed Dyslipidemia: A Multicenter, Randomized Trial
- PMID: 32904162
- PMCID: PMC7451794
- DOI: 10.1016/j.curtheres.2020.100595
Efficacy and Tolerability of a Fixed-Dose Combination of Rosuvastatin and Ezetimibe Compared with a Fixed-Dose Combination of Simvastatin and Ezetimibe in Brazilian Patients with Primary Hypercholesterolemia or Mixed Dyslipidemia: A Multicenter, Randomized Trial
Abstract
Background: The addition of ezetimibe to statin therapy has been reported to result in increased efficacy for reduction of LDL-C levels and achievement of lipid targets, compared with monotherapy.
Objective: This study was designed to demonstrate the noninferiority of therapy with fixed-dose rosuvastatin plus ezetimibe formulations versus fixed dose simvastatin and ezetimibe formulations for reduction of LDL-C levels in Brazilian patients with hypercholesterolemia or mixed dyslipidemia.
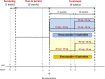
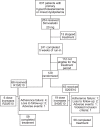
Methods: Phase III, multicenter, randomized, parallel, open-label, noninferiority study that included male and female participants (aged 21-80 years) with hypercholesterolemia or mixed dyslipidemia. After a 1-week screening period with washout of lipid-lowering medications when needed, patients were treated with simvastatin 20 mg/d for 5 weeks. Participants with LDL-C levels ≥100 mg/dL after the initial treatment were submitted to a 1-week washout period, and then randomized 1:1 to receive either combined rosuvastatin 10 mg + ezetimibe 10 mg (R/E) or simvastatin 20 mg + ezetimibe 10 mg (S/E) for 4 weeks and, if they still did not achieve the stipulated target, doses were readjusted to rosuvastatin 20 mg + ezetimibe 10 mg or simvastatin 40 mg + ezetimibe 10 mg, respectively, for 4 weeks.
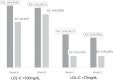
Results: One hundred twenty-nine participants were enrolled, including 66 in R/E and 63 in S/E. At the end of simvastatin 20 mg treatment period, mean LDL-C values were 124.79 mg/dL and 121.27 mg/dL for participants randomized to R/E and S/E arms, respectively. After 4 weeks of R/E 10 mg + 10 mg or S/E 20 mg + 10 mg combined treatments, adjusted mean LDL-C values were 74.21 mg/dL and 85.58 mg/dL, respectively (P = 0.0005), and after 9 weeks, with dose adjustment to R/E 20 mg + 10 mg in 6 patients and to S/E 40 mg +10 mg in 19 patients, LDL-C adjusted mean values were 75.29 mg/dL and 86.62 mg/dL, respectively (P = 0.0006). There was a statistically significant difference between the association R/E and S/E (P = 0.0013) in percentage change of LDL-C after 9 weeks of combined treatments. The adjusted mean difference was estimated at -10.32% (95% CI, -16.94% to -3.70%). The LDL-C <100 mg/dL target was achieved in a significantly greater proportion of participants at week 4 in the R/E compared with the S/E arm (84.8% vs 68.2%; P = .0257), and at week 9, the proportion was 81.2% versus 73.0%, respectively (P = 0.23). LDL-C <70 mg/dL was achieved at a significantly greater proportion in the R/E arm, both at week 4 (45.4% vs 15.9%; P = 0.003) and week 9 (40.9% vs 15.9%; P = 0.0017). A statistically significant difference at week 9 (P = 0.0106) was observed in fasting blood glucose in the R/E arm, but the overall incidence of adverse events was not significantly different between groups.
Conclusions: Rosuvastatin and ezetimibe fixed dose combination in both 10 mg/10 mg and 20 mg/10 mg doses, respectively, provided significantly lower levels of LDL-C compared with simvastatin and ezetimibe in doses of 20 mg/10 mg and 40 mg/10 mg, respectively. The fixed-dose combinations were both effective and well tolerated in this Brazilian study population. ClinicalTrials.gov identifier: NCT01420549. (Curr Ther Res Clin Exp. 2020; 81:XXX-XXX).
Keywords: Hypercholesterolemia; Rosuvastatin plus ezetimibe; Single-pill combination; Sinvastatin plus ezetimibe.
© 2020 The Author(s).
Figures
Similar articles
-
A Phase III, Multicenter, Randomized, Double-blind, Active Comparator Clinical Trial to Compare the Efficacy and Safety of Combination Therapy With Ezetimibe and Rosuvastatin Versus Rosuvastatin Monotherapy in Patients With Hypercholesterolemia: I-ROSETTE (Ildong Rosuvastatin & Ezetimibe for Hypercholesterolemia) Randomized Controlled Trial.Clin Ther. 2018 Feb;40(2):226-241.e4. doi: 10.1016/j.clinthera.2017.12.018. Clin Ther. 2018. PMID: 29402522 Clinical Trial.
-
Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial.JAMA. 2014 May 14;311(18):1870-82. doi: 10.1001/jama.2014.4030. JAMA. 2014. PMID: 24825642 Clinical Trial.
-
Impact of Switching From High-Efficacy Lipid-Lowering Therapies to Generic Simvastatin on LDL-C Levels and LDL-C Goal Attainment Among High-Risk Primary and Secondary Prevention Populations in the United Kingdom.Clin Ther. 2015 Apr 1;37(4):804-15. doi: 10.1016/j.clinthera.2014.12.019. Epub 2015 Jan 24. Clin Ther. 2015. PMID: 25626487
-
Rosuvastatin/Ezetimibe: A Review in Hypercholesterolemia.Am J Cardiovasc Drugs. 2020 Aug;20(4):381-392. doi: 10.1007/s40256-020-00421-1. Am J Cardiovasc Drugs. 2020. PMID: 32648167 Review.
-
Efficacy of Ezetimibe/Simvastatin (10/10 mg) versus High Dose Statin in Dyslipidemia Patients: A Meta-Analysis of Randomized Controlled Trials.Iran J Public Health. 2019 Aug;48(8):1405-1417. Iran J Public Health. 2019. PMID: 32292723 Free PMC article. Review.
Cited by
-
Real-World Evidence Evaluation on the Lipid Profile, Therapeutic Goals, and Safety of the Fixed-Dose Combination of Rosuvastatin/Ezetimibe (Trezete®) in Dyslipidemia Patients.Cardiol Res Pract. 2022 Sep 10;2022:9464733. doi: 10.1155/2022/9464733. eCollection 2022. Cardiol Res Pract. 2022. PMID: 36124294 Free PMC article.
-
A Prospective Randomized, Double-Blind, Multi-Center, Phase III Clinical Trial Evaluating the Efficacy and Safety of Olmesartan/Amlodipine plus Rosuvastatin Combination Treatment in Patients with Concomitant Hypertension and Dyslipidemia: A LEISURE Study.J Clin Med. 2022 Jan 11;11(2):350. doi: 10.3390/jcm11020350. J Clin Med. 2022. PMID: 35054044 Free PMC article.
-
Statin associated adverse reactions in Latin America: a scoping review.BMJ Open. 2021 Oct 1;11(10):e050675. doi: 10.1136/bmjopen-2021-050675. BMJ Open. 2021. PMID: 34598987 Free PMC article.
-
Hypercholesterolemia: a literature review on management using tafolecimab: a novel member of PCSK9 monoclonal antibodies.Ann Med Surg (Lond). 2024 Mar 12;86(5):2818-2827. doi: 10.1097/MS9.0000000000001945. eCollection 2024 May. Ann Med Surg (Lond). 2024. PMID: 38694324 Free PMC article. Review.
-
Is it Time for Single-Pill Combinations in Dyslipidemia?Am J Cardiovasc Drugs. 2022 May;22(3):239-249. doi: 10.1007/s40256-021-00498-2. Epub 2021 Sep 22. Am J Cardiovasc Drugs. 2022. PMID: 34549371 Free PMC article. Review.
References
-
- Klag MJ, Ford DE, Mead LA. Serum cholesterol in young men and subsequent cardiovascular disease. New England Journal of Medicine. 1993;328:313–318. - PubMed
-
- Lemieux L, Lamarche B, Couillard C. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Archives of internal medicine. 2001;161:2685–2692. - PubMed
-
- Sniderman AD, Williams K, Contois JH. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Cardiovascular Quality and Outcomes. 2011;4:337–345. - PubMed
-
- Verschuren WM, Jacobs DR, Bloemberg BP. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA. 1995;274:131–136. - PubMed
-
- Istvan E. Statin inhibition of HMG-CoA reductase: a 3-dimensional view. Atherosclerosis Supplements. 2003;4:3–8. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

