Predicting risk of late age-related macular degeneration using deep learning
- PMID: 32904246
- PMCID: PMC7453007
- DOI: 10.1038/s41746-020-00317-z
Predicting risk of late age-related macular degeneration using deep learning
Abstract
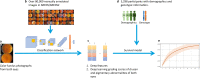
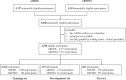
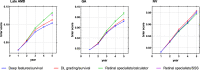
By 2040, age-related macular degeneration (AMD) will affect ~288 million people worldwide. Identifying individuals at high risk of progression to late AMD, the sight-threatening stage, is critical for clinical actions, including medical interventions and timely monitoring. Although deep learning has shown promise in diagnosing/screening AMD using color fundus photographs, it remains difficult to predict individuals' risks of late AMD accurately. For both tasks, these initial deep learning attempts have remained largely unvalidated in independent cohorts. Here, we demonstrate how deep learning and survival analysis can predict the probability of progression to late AMD using 3298 participants (over 80,000 images) from the Age-Related Eye Disease Studies AREDS and AREDS2, the largest longitudinal clinical trials in AMD. When validated against an independent test data set of 601 participants, our model achieved high prognostic accuracy (5-year C-statistic 86.4 (95% confidence interval 86.2-86.6)) that substantially exceeded that of retinal specialists using two existing clinical standards (81.3 (81.1-81.5) and 82.0 (81.8-82.3), respectively). Interestingly, our approach offers additional strengths over the existing clinical standards in AMD prognosis (e.g., risk ascertainment above 50%) and is likely to be highly generalizable, given the breadth of training data from 82 US retinal specialty clinics. Indeed, during external validation through training on AREDS and testing on AREDS2 as an independent cohort, our model retained substantially higher prognostic accuracy than existing clinical standards. These results highlight the potential of deep learning systems to enhance clinical decision-making in AMD patients.
Keywords: Eye manifestations; Image processing; Machine learning; Prognostic markers.
© This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures

Similar articles
-
A Deep Learning Algorithm for Prediction of Age-Related Eye Disease Study Severity Scale for Age-Related Macular Degeneration from Color Fundus Photography.Ophthalmology. 2018 Sep;125(9):1410-1420. doi: 10.1016/j.ophtha.2018.02.037. Epub 2018 Apr 10. Ophthalmology. 2018. PMID: 29653860
-
Age-related Macular Degeneration: Nutrition, Genes and Deep Learning-The LXXVI Edward Jackson Memorial Lecture.Am J Ophthalmol. 2020 Sep;217:335-347. doi: 10.1016/j.ajo.2020.05.042. Epub 2020 Jun 20. Am J Ophthalmol. 2020. PMID: 32574780 Free PMC article.
-
Assessment of Deep Generative Models for High-Resolution Synthetic Retinal Image Generation of Age-Related Macular Degeneration.JAMA Ophthalmol. 2019 Mar 1;137(3):258-264. doi: 10.1001/jamaophthalmol.2018.6156. JAMA Ophthalmol. 2019. PMID: 30629091 Free PMC article.
-
Biomarkers for the Progression of Intermediate Age-Related Macular Degeneration.Ophthalmol Ther. 2023 Dec;12(6):2917-2941. doi: 10.1007/s40123-023-00807-9. Epub 2023 Sep 29. Ophthalmol Ther. 2023. PMID: 37773477 Free PMC article. Review.
-
Prevention and treatment of age-related macular degeneration: an update for pharmacists.Consult Pharm. 2013 Nov;28(11):723-37. doi: 10.4140/TCP.n.2013.723. Consult Pharm. 2013. PMID: 24217192 Review.
Cited by
-
Harnessing the power of longitudinal medical imaging for eye disease prognosis using Transformer-based sequence modeling.ArXiv [Preprint]. 2024 Jul 30:arXiv:2405.08780v2. ArXiv. 2024. Update in: NPJ Digit Med. 2024 Aug 16;7(1):216. doi: 10.1038/s41746-024-01207-4. PMID: 39371086 Free PMC article. Updated. Preprint.
-
The Need for Artificial Intelligence Based Risk Factor Analysis for Age-Related Macular Degeneration: A Review.Diagnostics (Basel). 2022 Dec 30;13(1):130. doi: 10.3390/diagnostics13010130. Diagnostics (Basel). 2022. PMID: 36611422 Free PMC article. Review.
-
The Ethical and Societal Considerations for the Rise of Artificial Intelligence and Big Data in Ophthalmology.Front Med (Lausanne). 2022 Jun 28;9:845522. doi: 10.3389/fmed.2022.845522. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35836952 Free PMC article.
-
RNA-seq analysis reveals differentially expressed inflammatory chemokines in a rat retinal degeneration model induced by sodium iodate.J Int Med Res. 2022 Aug;50(8):3000605221119376. doi: 10.1177/03000605221119376. J Int Med Res. 2022. PMID: 36036255 Free PMC article.
-
Retinal pigment epithelium-Bruch's membrane volume in grading of age-related macular degeneration.Int J Ophthalmol. 2023 Nov 18;16(11):1827-1831. doi: 10.18240/ijo.2023.11.14. eCollection 2023. Int J Ophthalmol. 2023. PMID: 38028508 Free PMC article.
References
-
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

