Tramadol enhances PGF2α-stimulated osteoprotegerin synthesis in osteoblasts
- PMID: 32904295
- PMCID: PMC7452493
- DOI: 10.1016/j.heliyon.2020.e04779
Tramadol enhances PGF2α-stimulated osteoprotegerin synthesis in osteoblasts
Abstract
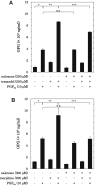
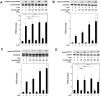
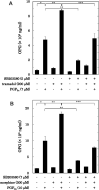
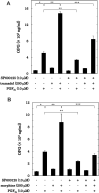
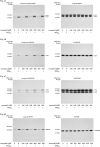
Osteoprotegerin (OPG) synthesized by osteoblasts is currently considered a crucial regulator to suppress the formation and function of osteoclasts. We previously showed that the synthesis of OPG is stimulated by prostaglandin F2α (PGF2α) in the involvement of p38 mitogen-activated protein kinase (MAPK), stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) and p44/p42 MAPK in osteoblast-like MC3T3-E1 cells. We also found that Rho-kinase is involved in the signaling of PGF2α upstream of p38 MAPK in these cells. Tramadol is widely used to treat chronic pain, such as low back pain associated with osteoporosis. We investigated whether or not tramadol affects the OPG release induced by PGF2α in osteoblast-like MC3T3-E1 cells. The levels of OPG in the conditioned medium were measured by an enzyme-linked immunosorbent assay. The mRNA expression of OPG was determined with real-time reverse transcription polymerase chain reaction. The phosphorylation of target protein was determined with a Western blot analysis. PGF2α induced the release and the mRNA expression of OPG, which tramadol significantly enhanced. Morphine, a selective μ-opioid receptor (MOR) agonist, also enhanced the PGF2α-induced OPG release. In addition, naloxone, a MOR antagonist, suppressed the enhancement by tramadol or morphine of the PGF2α-induced OPG synthesis. Tramadol upregulated the phosphorylation of SAPK/JNK and p38 MAPK stimulated by PGF2α but not that of p44/p42 MAPK or myosin phosphatase targeting protein (MYPT), a substrate of Rho-kinase. The inhibitors of both p38 MAPK and SAPK/JNK, SB203580 and SP600125, respectively, reduced the tramadol amplification of OPG release stimulated by PGF2α. The present results strongly suggest that tramadol enhances the synthesis of OPG stimulated by PGF2α through MOR in osteoblasts, and that the amplifying effect is exerted at upstream of p38 MAPK and SAPK/JNK but downstream of Rho-kinase.
Keywords: Biochemistry; Bone; Cell biology; MAP kinase; Osteoblast.; Osteoprotegerin; PGF2α; Pain management; Pharmaceutical chemistry; Pharmaceutical science; Pharmacology; Tramadol; μ-opioid receptor.
© 2020 The Author(s).
Figures



Similar articles
-
Amplification by tramadol of PGD2-induced osteoprotegerin synthesis in osteoblasts: Involvement of μ-opioid receptor and 5-HT transporter.Prostaglandins Leukot Essent Fatty Acids. 2021 Sep;172:102323. doi: 10.1016/j.plefa.2021.102323. Epub 2021 Aug 8. Prostaglandins Leukot Essent Fatty Acids. 2021. PMID: 34392133
-
Acetaminophen reduces osteoprotegerin synthesis stimulated by PGE2 and PGF2α in osteoblasts: attenuation of SAPK/JNK but not p38 MAPK or p44/p42 MAPK.Biomed Res. 2021;42(2):77-84. doi: 10.2220/biomedres.42.77. Biomed Res. 2021. PMID: 33840687
-
Suppression by resveratrol of prostaglandin D2-stimulated osteoprotegerin synthesis in osteoblasts.Prostaglandins Leukot Essent Fatty Acids. 2014 Sep;91(3):73-80. doi: 10.1016/j.plefa.2014.04.003. Epub 2014 Apr 24. Prostaglandins Leukot Essent Fatty Acids. 2014. PMID: 24813642
-
Resveratrol suppresses prostaglandin F(2α)-induced osteoprotegerin synthesis in osteoblasts: inhibition of the MAP kinase signaling.Arch Biochem Biophys. 2014 Jan 15;542:39-45. doi: 10.1016/j.abb.2013.12.002. Epub 2013 Dec 11. Arch Biochem Biophys. 2014. PMID: 24333336
-
Resveratrol reduces prostaglandin E1-stimulated osteoprotegerin synthesis in osteoblasts: suppression of stress-activated protein kinase/c-Jun N-terminal kinase.Prostaglandins Other Lipid Mediat. 2015 Jan-Mar;116-117:57-63. doi: 10.1016/j.prostaglandins.2015.01.003. Epub 2015 Feb 9. Prostaglandins Other Lipid Mediat. 2015. PMID: 25677506
Cited by
-
Prostaglandin F2α Regulates Adipogenesis by Modulating Extracellular Signal-Regulated Kinase Signaling in Graves' Ophthalmopathy.Int J Mol Sci. 2023 Apr 10;24(8):7012. doi: 10.3390/ijms24087012. Int J Mol Sci. 2023. PMID: 37108173 Free PMC article.
References
-
- Agas D., Marchetti L., Hurley M.M., Sabbieti M.G. Prostaglandin F2α: a bone remodeling mediator. J. Cell. Physiol. 2013 - PubMed
-
- Boyce B.F., Rosenberg E., de Papp A.E., Duong L.T. The osteoclast, bone remodelling and treatment of metabolic bone disease. Eur. J. Clin. Invest. 2012 - PubMed
-
- Bravo L., Mico J.A., Berrocoso E. Discovery and development of tramadol for the treatment of pain. Expet Opin. Drug Discov. 2017 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

