Parametric nasopharyngeal swab for sampling COVID-19 and other respiratory viruses: Open source design, SLA 3-D printing and UV curing system
- PMID: 32904317
- PMCID: PMC7455530
- DOI: 10.1016/j.ohx.2020.e00135
Parametric nasopharyngeal swab for sampling COVID-19 and other respiratory viruses: Open source design, SLA 3-D printing and UV curing system
Erratum in
-
Erratum regarding previously published articles.HardwareX. 2021 Sep 22;10:e00235. doi: 10.1016/j.ohx.2021.e00235. eCollection 2021 Oct. HardwareX. 2021. PMID: 35607661 Free PMC article.
Abstract
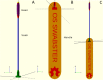
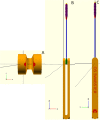
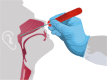
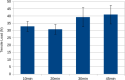
Access to nasopharyngeal swabs for sampling remain a bottleneck in some regions for COVID-19 testing. This study develops a distributed manufacturing solution using only an open source manufacturing tool chain consisting of two types of open source 3-D printing and batch UV curing, and provides a parametric fully free design of a nasopharyngeal swab. The swab was designed using parametric OpenSCAD in two components (a head with engineered break point and various handles), which has several advantages: i) minimizing print time on relatively slow SLA printers, ii) enabling the use of smaller print volume open source SLA printers, iii) reducing the amount of relatively expensive UV resin, and iv) enabling production of handle on more accessible material extrusion 3-D printers. A modular open source UV LED box was designed, fabricated for $45 and tested for batch curing. Swabs can be fabricated for $0.06-$0.12/swab. The results of the mechanical validation tests showed that the swabs could withstand greater forces than would be expected in normal clinical use. The swabs were also able to absorb a significant amounts of synthetic mucus materials and passed abrasion and handling tests. The results show the open source swab are promising candidates for clinical trials.
Keywords: 3-D printing; Additive manufacturing; COVID-19; Medical hardware; Nasal swab; Nasopharyngeal swab; Open hardware; RepRap; SLA; UV curing.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


























References
-
- World Health Organization, 2020. Critical preparedness, readiness and response actions for COVID-19: interim guidance, 7 March 2020 (No. WHO/COVID-19/Community_Actions/2020.1). World Health Organization.
-
- Silv, M., COVID-19: too little, too late?. The Lancet. doi: 10.1016/S0140-6736(20)30522-5
-
- L. Ramsey, Hospitals could be overwhelmed with patients and run out of beds and ventilators as the coronavirus pushes the US healthcare system to its limits. Mar 11, 2020, 8:53 AM Business Insider. https://www.businessinsider.com/coronavirus-intensive-care-unit-shortage....
-
- Rubinson L., Vaughn F., Nelson S., Giordano S., Kallstrom T., Buckley T., Burney T., Hupert N., Mutter R., Handrigan M., Yeskey K. Mechanical ventilators in US acute care hospitals. Disaster Med. Public Health Preparedness. 2010;4(3):199–206. - PubMed
LinkOut - more resources
Full Text Sources

