MicroRNA-421 Inhibits Apoptosis by Downregulating Caspase-3 in Human Colorectal Cancer
- PMID: 32904410
- PMCID: PMC7455595
- DOI: 10.2147/CMAR.S255787
MicroRNA-421 Inhibits Apoptosis by Downregulating Caspase-3 in Human Colorectal Cancer
Abstract
Purpose: Dysregulated microRNAs (miRNAs/miRs) have been reported to play significant roles in pathogenesis of colorectal cancer (CRC). Previous studies have demonstrated that miR-421 regulates apoptosis in some cancers. Caspase-3 plays a key role in apoptosis, but the relationship between miR-421 and caspase-3 in CRC has not been determined. In this study, we investigated the role of miR-421 in CRC and the relationship between miR-421 and caspase-3.
Methods: Expression of miR-421 and caspase-3 were detected in human paired CRC cancer tissues and corresponding paracancerous tissues. In situ detection of tissue, apoptosis was performed via the TUNEL assay. HCT116 and SW480 cell lines were subjected to several in vitro experiments to explore the relationship between miRNA421 and caspase-3 during apoptosis using miR421 mimics/antagomir and luciferase reporter assay. Apoptosis was measured by determining the levels and activity of caspase-3 as well as DNA fragmentation. Luciferase reporter assay was performed to determine the potential interaction of miR-421 with caspase-3.
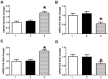
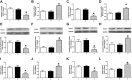
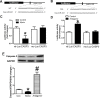
Results: The results showed that the expression of miR-421 in cancer tissues was higher than that in corresponding paracancerous tissues. Inhibition of miR-421 induced apoptosis, as shown by the upregulation of caspase-3 activity and expression as well as DNA fragmentation, which were attenuated by miR-421 mimic. We further showed that miR-421 targeted and inhibited CASP3 expression by targeting sites located in the 3'-untranslated region (3'-UTR) of CASP3 mRNA.
Conclusion: This study demonstrated an anti-apoptotic role of miR-421 in CRC and identified caspase-3 gene as a direct target of miR-421. These findings provide a potential treatment strategy using miR-421 as a therapeutic target for CRC.
Keywords: apoptosis; caspase-3; colorectal cancer; microRNA-421.
© 2020 Zhou et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
MicroRNA-215 Regulates the Apoptosis of HCT116 Colon Cancer Cells by Inhibiting X-Linked Inhibitor of Apoptosis Protein.Cancer Biother Radiopharm. 2021 Nov;36(9):728-736. doi: 10.1089/cbr.2019.3011. Epub 2020 May 28. Cancer Biother Radiopharm. 2021. PMID: 32460520
-
MicroRNA-124-3p suppresses PD-L1 expression and inhibits tumorigenesis of colorectal cancer cells via modulating STAT3 signaling.J Cell Physiol. 2021 Oct;236(10):7071-7087. doi: 10.1002/jcp.30378. Epub 2021 Apr 5. J Cell Physiol. 2021. PMID: 33821473
-
miR-107 Promotes Proliferation and Inhibits Apoptosis of Colon Cancer Cells by Targeting Prostate Apoptosis Response-4 (Par4).Oncol Res. 2017 Jul 5;25(6):967-974. doi: 10.3727/096504016X14803476672380. Epub 2016 Nov 29. Oncol Res. 2017. PMID: 27938501 Free PMC article.
-
GDPD5, a target of miR-195-5p, is associated with metastasis and chemoresistance in colorectal cancer.Biomed Pharmacother. 2018 May;101:945-952. doi: 10.1016/j.biopha.2018.03.028. Epub 2018 Mar 22. Biomed Pharmacother. 2018. PMID: 29635904
-
MicroRNA-218 inhibits cell cycle progression and promotes apoptosis in colon cancer by downregulating BMI1 polycomb ring finger oncogene.Mol Med. 2013 Feb 8;18(1):1491-8. doi: 10.2119/molmed.2012.00304. Mol Med. 2013. PMID: 23255074 Free PMC article.
Cited by
-
Global Perspectives on Coronary Artery Disease: The Emerging Role of miRNAs.Curr Atheroscler Rep. 2025 Jun 17;27(1):66. doi: 10.1007/s11883-025-01309-8. Curr Atheroscler Rep. 2025. PMID: 40526174 Review.
-
Crosstalk between non-coding RNAs and programmed cell death in colorectal cancer: implications for targeted therapy.Epigenetics Chromatin. 2025 Jan 15;18(1):3. doi: 10.1186/s13072-024-00560-8. Epigenetics Chromatin. 2025. PMID: 39810224 Free PMC article. Review.
-
Recent advances in the diagnostic and therapeutic roles of microRNAs in colorectal cancer progression and metastasis.Front Oncol. 2022 Oct 13;12:911856. doi: 10.3389/fonc.2022.911856. eCollection 2022. Front Oncol. 2022. PMID: 36313731 Free PMC article. Review.
-
circ_0000567/miR-421/TMEM100 Axis Promotes the Migration and Invasion of Lung Adenocarcinoma and Is Associated with Prognosis.J Cancer. 2022 Feb 28;13(5):1540-1552. doi: 10.7150/jca.60124. eCollection 2022. J Cancer. 2022. PMID: 35371319 Free PMC article.
-
Aberrant Post-Transcriptional Regulation of Protein Expression in the Development of Chronic Obstructive Pulmonary Disease.Int J Mol Sci. 2021 Nov 4;22(21):11963. doi: 10.3390/ijms222111963. Int J Mol Sci. 2021. PMID: 34769392 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

