Systematic analysis of nuclear gene function in respiratory growth and expression of the mitochondrial genome in S. cerevisiae
- PMID: 32904421
- PMCID: PMC7453639
- DOI: 10.15698/mic2020.09.729
Systematic analysis of nuclear gene function in respiratory growth and expression of the mitochondrial genome in S. cerevisiae
Abstract
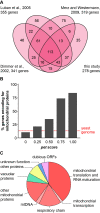
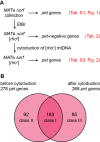
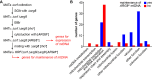
The production of metabolic energy in form of ATP by oxidative phosphorylation depends on the coordinated action of hundreds of nuclear-encoded mitochondrial proteins and a handful of proteins encoded by the mitochondrial genome (mtDNA). We used the yeast Saccharomyces cerevisiae as a model system to systematically identify the genes contributing to this process. Integration of genome-wide high-throughput growth assays with previously published large data sets allowed us to define with high confidence a set of 254 nuclear genes that are indispensable for respiratory growth. Next, we induced loss of mtDNA in the yeast deletion collection by growth on ethidium bromide-containing medium and identified twelve genes that are essential for viability in the absence of mtDNA (i.e. petite-negative). Replenishment of mtDNA by cytoduction showed that respiratory-deficient phenotypes are highly variable in many yeast mutants. Using a mitochondrial genome carrying a selectable marker, ARG8 m , we screened for mutants that are specifically defective in maintenance of mtDNA and mitochondrial protein synthesis. We found that up to 176 nuclear genes are required for expression of mitochondria-encoded proteins during fermentative growth. Taken together, our data provide a comprehensive picture of the molecular processes that are required for respiratory metabolism in a simple eukaryotic cell.
Keywords: mitochondria; mitochondrial DNA; oxidative phosphorylation; petite mutant; yeast.
Copyright: © 2020 Stenger et al.
Conflict of interest statement
Conflict of interest: The authors declare no conflicts of interest.
Figures
Similar articles
-
Genome-wide deletion mutant analysis reveals genes required for respiratory growth, mitochondrial genome maintenance and mitochondrial protein synthesis in Saccharomyces cerevisiae.Genome Biol. 2009;10(9):R95. doi: 10.1186/gb-2009-10-9-r95. Epub 2009 Sep 14. Genome Biol. 2009. PMID: 19751518 Free PMC article.
-
Adaptive expression responses in the Pol-gamma null strain of S. pombe depleted of mitochondrial genome.BMC Genomics. 2007 Sep 15;8:323. doi: 10.1186/1471-2164-8-323. BMC Genomics. 2007. PMID: 17868468 Free PMC article.
-
Growth of eukaryotic cells in relation to the structure of mitochondrial membranes and mitochondrial genome.Folia Microbiol (Praha). 1999;44(6):697-702. doi: 10.1007/BF02825665. Folia Microbiol (Praha). 1999. PMID: 11097029
-
Genetic control of oxidative phosphorylation and experimental models of defects.Hum Reprod. 2000 Jul;15 Suppl 2:18-27. doi: 10.1093/humrep/15.suppl_2.18. Hum Reprod. 2000. PMID: 11041510 Review.
-
The petite mutation in yeasts: 50 years on.Int Rev Cytol. 2000;194:197-238. doi: 10.1016/s0074-7696(08)62397-9. Int Rev Cytol. 2000. PMID: 10494627 Review.
Cited by
-
Construction and iterative redesign of synXVI a 903 kb synthetic Saccharomyces cerevisiae chromosome.Nat Commun. 2025 Jan 20;16(1):841. doi: 10.1038/s41467-024-55318-3. Nat Commun. 2025. PMID: 39833175 Free PMC article.
-
Saccharomyces paradoxus Transcriptional Alterations in Cells of Distinct Phenotype and Viral dsRNA Content.Microorganisms. 2020 Nov 30;8(12):1902. doi: 10.3390/microorganisms8121902. Microorganisms. 2020. PMID: 33266158 Free PMC article.
-
A genome-wide copper-sensitized screen identifies novel regulators of mitochondrial cytochrome c oxidase activity.J Biol Chem. 2021 Jan-Jun;296:100485. doi: 10.1016/j.jbc.2021.100485. Epub 2021 Mar 1. J Biol Chem. 2021. PMID: 33662401 Free PMC article.
-
Mapping mitonuclear epistasis using a novel recombinant yeast population.PLoS Genet. 2023 Mar 29;19(3):e1010401. doi: 10.1371/journal.pgen.1010401. eCollection 2023 Mar. PLoS Genet. 2023. PMID: 36989278 Free PMC article.
-
Pathways shaping the mitochondrial inner membrane.Open Biol. 2021 Dec;11(12):210238. doi: 10.1098/rsob.210238. Epub 2021 Dec 1. Open Biol. 2021. PMID: 34847778 Free PMC article. Review.
References
-
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429(6990):417–423. doi: 10.1038/nature02517. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
