Microbiota Supplementation with Bifidobacterium and Lactobacillus Modifies the Preterm Infant Gut Microbiota and Metabolome: An Observational Study
- PMID: 32904427
- PMCID: PMC7453906
- DOI: 10.1016/j.xcrm.2020.100077
Microbiota Supplementation with Bifidobacterium and Lactobacillus Modifies the Preterm Infant Gut Microbiota and Metabolome: An Observational Study
Abstract
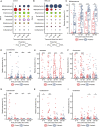
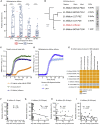
Supplementation with members of the early-life microbiota as "probiotics" is increasingly used in attempts to beneficially manipulate the preterm infant gut microbiota. We performed a large observational longitudinal study comprising two preterm groups: 101 infants orally supplemented with Bifidobacterium and Lactobacillus (Bif/Lacto) and 133 infants non-supplemented (control) matched by age, sex, and delivery method. 16S rRNA gene profiling on fecal samples (n = 592) showed a predominance of Bifidobacterium and a lower abundance of pathobionts in the Bif/Lacto group. Metabolomic analysis showed higher fecal acetate and lactate and a lower fecal pH in the Bif/Lacto group compared to the control group. Fecal acetate positively correlated with relative abundance of Bifidobacterium, consistent with the ability of the supplemented Bifidobacterium strain to metabolize human milk oligosaccharides into acetate. This study demonstrates that microbiota supplementation is associated with a Bifidobacterium-dominated preterm microbiota and gastrointestinal environment more closely resembling that of full-term infants.
Keywords: Bifidobacterium; Lactobacillus; human milk oligosaccharides; metabolites; microbiota; pH; pathobionts; preterm infant; probiotic; supplementation.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- World Health Organization . 2019. Preterm birth.https://www.who.int/news-room/fact-sheets/detail/preterm-birth Accessed September 25, 2019.
-
- Mulder I.E., Schmidt B., Lewis M., Delday M., Stokes C.R., Bailey M., Aminov R.I., Gill B.P., Pluske J.R., Mayer C.D., Kelly D. Restricting microbial exposure in early life negates the immune benefits associated with gut colonization in environments of high microbial diversity. PLoS ONE. 2011;6:e28279. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- BBS/E/F/00044409/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10353/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10356/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

