Effect of Electroacupuncture at GV20 on Sleep Deprivation-Induced Depression-Like Behavior in Mice
- PMID: 32904512
- PMCID: PMC7456497
- DOI: 10.1155/2020/7481813
Effect of Electroacupuncture at GV20 on Sleep Deprivation-Induced Depression-Like Behavior in Mice
Abstract
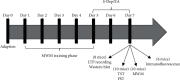
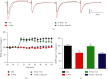
Accumulating evidence suggests that sleep deprivation (S-Dep) is a critical risk factor for depression. Electroacupuncture (EA) treatment has been reported to ameliorate posttraumatic stress disorder- (PSTD-) like behavior and enhance hippocampal neurogenesis. However, whether EA treatment has any beneficial effect on S-Dep-induced depression-like behavior is still unknown. In the present study, we focused on whether EA at Baihui (GV20) can ameliorate the deterioration effect of S-Dep in mice. Mice were randomly divided into normal, S-Dep, S-Dep + EA, and S-Dep + sham EA groups. Cognitive behavior test and in vitro assay were performed separately to avoid the influence of behavior test on synaptic transmission and protein expression. Depression-like behaviors were determined by forced swimming test (FST), tail suspension test (TST), and Morris water maze (MWM). Neurogenesis was identified by BrdU, DCX, and NeuN immunofluorescence staining. In vitro long-term potentiation was detected by high frequency stimulation (HFS) at Schaffer collateral-CA1 synapses in hippocampal slices. Brain-derived neurotropic factor (BDNF) and tropomyosin receptor kinase B (TrkB) protein expression level were assayed by western blot. Our results indicated that D-Sep mice demonstrated depression-like behaviors determined by prolonged immobility duration in FST and TST; D-Sep mice also manifested spatial memory retention deficit in MWM. Furthermore, EA treatment ameliorated D-Sep-induced depression-like behaviors and spatial memory retention deficit. Mechanically, EA treatment alleviated neuron progenitor cell proliferation and differentiation, ameliorated the field excitatory postsynaptic potentials (fEPSPs) slope impaired by S-Dep, and elevated BDNF/TrkB protein expression. Taken together, our data suggested that EA treatment has a protective effect on S-Dep-induced depression-like behavior and cognitive impairment, which may be through regulating BDNF/TrkB protein expression.
Copyright © 2020 Xiaohong Xu et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures





Similar articles
-
Efficacy of electroacupuncture stimulating Shenmen (HT7), Baihui (GV20), Sanyinjiao (SP6) on spatial learning and memory deficits in rats with insomnia induced by para-chlorophenylalanine: a single acupoint combined acupoints.J Tradit Chin Med. 2023 Aug;43(4):704-714. doi: 10.19852/j.cnki.jtcm.20230308.001. J Tradit Chin Med. 2023. PMID: 37454255 Free PMC article.
-
Possible antidepressant effects and mechanism of electroacupuncture in behaviors and hippocampal synaptic plasticity in a depression rat model.Brain Res. 2015 Dec 10;1629:291-7. doi: 10.1016/j.brainres.2015.10.033. Epub 2015 Oct 23. Brain Res. 2015. PMID: 26505920
-
Electroacupuncture restores hippocampal synaptic plasticity via modulation of 5-HT receptors in a rat model of depression.Brain Res Bull. 2018 May;139:256-262. doi: 10.1016/j.brainresbull.2018.03.004. Epub 2018 Mar 7. Brain Res Bull. 2018. PMID: 29524471
-
Differential effects of citalopram on sleep-deprivation-induced depressive-like behavior and memory impairments in mice.Prog Neuropsychopharmacol Biol Psychiatry. 2019 Jan 10;88:102-111. doi: 10.1016/j.pnpbp.2018.07.013. Epub 2018 Jul 12. Prog Neuropsychopharmacol Biol Psychiatry. 2019. PMID: 30017777
-
[Effect of electroacupuncture on cyclin-dependent kinase 5 and Tau protein in hippocampus of SAMP8 mice].Zhen Ci Yan Jiu. 2020 Jul 25;45(7):529-34. doi: 10.13702/j.1000-0607.190752. Zhen Ci Yan Jiu. 2020. PMID: 32705825 Chinese.
Cited by
-
Effect of acupuncture on cognitive impairment induced by sleep deprivation in animal models: a preclinical systematic review and meta-analysis.Front Aging Neurosci. 2025 Mar 19;17:1560032. doi: 10.3389/fnagi.2025.1560032. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40177248 Free PMC article.
-
Acupuncture ameliorates breast cancer-related fatigue by regulating the gut microbiota-gut-brain axis.Front Endocrinol (Lausanne). 2022 Aug 24;13:921119. doi: 10.3389/fendo.2022.921119. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36093113 Free PMC article.
-
Electroacupuncture prevents astrocyte atrophy to alleviate depression.Cell Death Dis. 2023 May 29;14(5):343. doi: 10.1038/s41419-023-05839-4. Cell Death Dis. 2023. PMID: 37248211 Free PMC article.
-
Beneficial effect of electroacupuncture on the distribution of foreign substances in the brain of rats developing depression-like behavior.IBRO Neurosci Rep. 2023 Mar 28;14:398-406. doi: 10.1016/j.ibneur.2023.03.014. eCollection 2023 Jun. IBRO Neurosci Rep. 2023. PMID: 37388496 Free PMC article.
-
Electroacupuncture alleviated depression-like behaviors in ventromedial prefrontal cortex of chronic unpredictable mild stress-induced rats: Increasing synaptic transmission and phosphorylating dopamine transporter.CNS Neurosci Ther. 2023 Sep;29(9):2608-2620. doi: 10.1111/cns.14200. Epub 2023 Apr 1. CNS Neurosci Ther. 2023. PMID: 37002793 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

