Evaluating Daratumumab in the Treatment of Multiple Myeloma: Safety, Efficacy and Place in Therapy
- PMID: 32904669
- PMCID: PMC7457558
- DOI: 10.2147/CMAR.S212526
Evaluating Daratumumab in the Treatment of Multiple Myeloma: Safety, Efficacy and Place in Therapy
Abstract
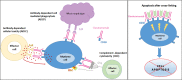
Despite the tremendous advances in the treatment of multiple myeloma, mortality remains significant, highlighting the need for new effective strategies. In recent years, daratumumab, a novel human monoclonal antibody, binding CD38, has dramatically improved outcomes either as monotherapy or in combination with traditional regimens. Originally approved for relapsed/refractory multiple myeloma, this breakthrough medication is now being used as frontline therapy in patients with newly diagnosed multiple myeloma regardless of transplant eligibility, with trials showing promising results. Its tolerable side-effect profile and enhanced efficacy have led to its widespread incorporation into the management of multiple myeloma and further exploration about its use in other entities such as smoldering myeloma, MGUS, MGRS and amyloidosis. This comprehensive review will discuss daratumumab's mechanism of action and safety profile, as well as research which has defined its current approved indications, and ongoing clinical investigation that will define its future.
Keywords: daratumumab; newly diagnosed multiple myeloma; relapsed/refractory multiple myeloma.
© 2020 Dima et al.
Conflict of interest statement
Dr Danai Dima and Dr Joshua Dower are co-first authors. Dr Raymond Comenzo reports a patent WO2016187546A1 issued. The authors report no other conflicts of interest in this work.
References
-
- Surveillance, Epidemiology, and End Results (SEER) Program. Cancer stat facts: myeloma. - PubMed
-
- Stege CAM, Nasserinejad K, Levin M-D, et al. Efficacy and tolerability of Ixazomib, Daratumumab and Low Dose Dexamethasone (IDd) in Unfit and Frail Newly Diagnosed Multiple Myeloma (NDMM) patients; first interim safety analysis of the Phase II HOVON 143 Study. Blood. 2018;132(Supplement 1):596. doi:10.1182/blood-2018-99-114668 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials