Maximum bite force in patients with spinal muscular atrophy during the first year of nusinersen therapy - A pilot study
- PMID: 32904902
- PMCID: PMC7460731
- DOI: 10.36185/2532-1900-010
Maximum bite force in patients with spinal muscular atrophy during the first year of nusinersen therapy - A pilot study
Abstract
Objectives: Spinal muscular atrophy is a monogenic disease characterized by progressive spinal and bulbar muscle weakness and atrophy. It is caused by the degeneration of alpha-motoneurons. The recent approval of the antisense oligonucleotide nusinersen highlights the need for reliable clinical tools to evaluate motor function in patients with neuromuscular disorders. Measurement of the bulbar neuromuscular function (e.g., bite force) could be an extension to existing motor scales, sensitive to more nuanced changes, especially in symptomatic patients with severely reduced functional abilities.
Materials and methods: Maximum bite force measurement was used to quantify changes of the masticatory function in adult monozygotic female twins with SMA type II. Using piezoelectric transducers, 550 observations were recorded for each patient during the first year of nusinersen therapy.
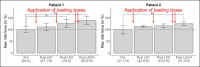
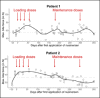
Results: During the application of four loading doses of nusinersen, bite force levels steadily increased and reached a statistically significantly higher level compared to the initial state in both patients. Subsequent maintenance doses coincided with smaller or no statistically significant changes in maximum bite force.
Conclusions: This pilot study indicates that the measurement of maximum bite force may be a useful tool to detect changes of the bulbar function in SMA patients. As such, it may supplement existing scales to identify treatment-related changes in motor function.
Keywords: antisense oligonucleotide; masticatory force; motor neurons; spinal muscular atrophy.
©2020 Gaetano Conte Academy - Mediterranean Society of Myology, Naples, Italy.
Figures

Similar articles
-
The Maximum Bite Force for Treatment Evaluation in Severely Affected Adult SMA Patients-Protocol for a Longitudinal Study.Front Neurol. 2020 Feb 25;11:139. doi: 10.3389/fneur.2020.00139. eCollection 2020. Front Neurol. 2020. PMID: 32161570 Free PMC article.
-
Effect of nusinersen on motor, respiratory and bulbar function in early-onset spinal muscular atrophy.Brain. 2023 Feb 13;146(2):668-677. doi: 10.1093/brain/awac252. Brain. 2023. PMID: 35857854
-
Nusinersen for Spinal Muscular Atrophy in the United States: Findings From a Retrospective Claims Database Analysis.Adv Ther. 2021 Dec;38(12):5809-5828. doi: 10.1007/s12325-021-01938-w. Epub 2021 Oct 28. Adv Ther. 2021. PMID: 34713391 Free PMC article.
-
Changing respiratory expectations with the new disease trajectory of nusinersen treated spinal muscular atrophy [SMA] type 1.Paediatr Respir Rev. 2018 Sep;28:11-17. doi: 10.1016/j.prrv.2018.07.002. Epub 2018 Jul 12. Paediatr Respir Rev. 2018. PMID: 30414815 Review.
-
Drug treatment for spinal muscular atrophy types II and III.Cochrane Database Syst Rev. 2020 Jan 6;1(1):CD006282. doi: 10.1002/14651858.CD006282.pub5. Cochrane Database Syst Rev. 2020. PMID: 32006461 Free PMC article.
Cited by
-
Risdiplam improves subjective swallowing quality in non-ambulatory adult patients with 5q-spinal muscular atrophy despite advanced motor impairment.J Neurol. 2024 May;271(5):2649-2657. doi: 10.1007/s00415-024-12203-9. Epub 2024 Feb 15. J Neurol. 2024. PMID: 38358553 Free PMC article.
-
Objective measurement of oral function in adults with spinal muscular atrophy.Orphanet J Rare Dis. 2023 May 3;18(1):103. doi: 10.1186/s13023-023-02688-4. Orphanet J Rare Dis. 2023. PMID: 37138365 Free PMC article.
-
Development of an International SMA Bulbar Assessment for Inter-professional Administration.J Neuromuscul Dis. 2023;10(4):639-652. doi: 10.3233/JND-221672. J Neuromuscul Dis. 2023. PMID: 37212069 Free PMC article.
-
Spinal Muscular Atrophy after Nusinersen Therapy: Improved Physiology in Pediatric Patients with No Significant Change in Urine, Serum, and Liquor 1H-NMR Metabolomes in Comparison to an Age-Matched, Healthy Cohort.Metabolites. 2021 Mar 30;11(4):206. doi: 10.3390/metabo11040206. Metabolites. 2021. PMID: 33808177 Free PMC article.
-
Bite Force Transducers and Measurement Devices.Front Bioeng Biotechnol. 2021 Apr 9;9:665081. doi: 10.3389/fbioe.2021.665081. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33898409 Free PMC article. Review.
References
-
- Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: Part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord 2018;28:103-15. https://doi.org/10.1016/j.nmd.2017.11.005 10.1016/j.nmd.2017.11.005 - DOI - PubMed
-
- Lefebvre S, Bürglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995;80:155-65. https://doi.org/10.1016/0092-8674(95)90460-3 10.1016/0092-8674(95)90460-3 - DOI - PubMed
-
- Wirth B, Herz M, Wetter A, et al. Quantitative analysis of survival motor neuron copies: identification of subtle SMN1 mutations in patients with spinal muscular atrophy, genotype-phenotype correlation, and implications for genetic counseling. Am J Hum Genet 1999;64:1340-56. https://doi.org/10.1086/302369 10.1086/302369 - DOI - PMC - PubMed
-
- Munsat TL, Davies KE. International SMA consortium meeting. (26-28 June 1992, Bonn, Germany). Neuromuscul Disord 1992;2:423-8. https://doi.org/10.1016/S0960-8966(06)80015-5 10.1016/S0960-8966(06)80015-5 - DOI - PubMed
-
- Dubowitz V. Chaos in the classification of SMA: a possible resolution. Neuromuscul Disord 1995;5:3-5. https://doi.org/10.1016/0960-8966(94)00075-K 10.1016/0960-8966(94)00075-K - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
