Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory
- PMID: 32904944
- PMCID: PMC7461279
- DOI: 10.1002/jbm4.10405
Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory
Abstract
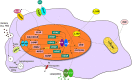
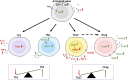
Regulation of immune function continues to be one of the most well-recognized extraskeletal actions of vitamin D. This stemmed initially from the discovery that antigen presenting cells such as macrophages could actively metabolize precursor 25-hydroxyvitamin D (25D) to active 1,25-dihydroxyvitamin D (1,25D). Parallel observation that activated cells from the immune system expressed the intracellular vitamin D receptor (VDR) for 1,25D suggested a potential role for vitamin D as a localized endogenous modulator of immune function. Subsequent studies have expanded our understanding of how vitamin D exerts effects on both the innate and adaptive arms of the immune system. At an innate level, intracrine synthesis of 1,25D by macrophages and dendritic cells stimulates expression of antimicrobial proteins such as cathelicidin, as well as lowering intracellular iron concentrations via suppression of hepcidin. By potently enhancing autophagy, 1,25D may also play an important role in combatting intracellular pathogens such as M. tuberculosis and viral infections. Local synthesis of 1,25D by macrophages and dendritic cells also appears to play a pivotal role in mediating T-cell responses to vitamin D, leading to suppression of inflammatory T helper (Th)1 and Th17 cells, and concomitant induction of immunotolerogenic T-regulatory responses. The aim of this review is to provide an update on our current understanding of these prominent immune actions of vitamin D, as well as highlighting new, less well-recognized immune effects of vitamin D. The review also aims to place this mechanistic basis for the link between vitamin D and immunity with studies in vivo that have explored a role for vitamin D supplementation as a strategy for improved immune health. This has gained prominence in recent months with the global coronavirus disease 2019 health crisis and highlights important new objectives for future studies of vitamin D and immune function. © 2020 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: ANTIBACTERIAL; AUTOPHAGY; CATHELICIDIN; HEPCIDIN; INFLAMMATION; NOD2; Th1; Th17; Treg; VITAMIN D; β‐DEFENSIN 2.
© 2020 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Figures
References
-
- Tavera‐Mendoza LE, White JH. Cell defenses and the sunshine vitamin. Sci Am. 2007;297(5):62–5. - PubMed
-
- Shinkyo R, Sakaki T, Kamakura M, Ohta M, Inouye K. Metabolism of vitamin D by human microsomal CYP2R1. Biochem Biophys Res Commun. 2004;324(1):451–7. - PubMed
-
- Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25‐hydroxyvitamin d(3)‐1 alpha‐hydroxylase. J Clin Endocrinol Metab. 2001;86(2):888–94. - PubMed
LinkOut - more resources
Full Text Sources
