Patient-derived cells from recurrent tumors that model the evolution of IDH-mutant glioma
- PMID: 32904945
- PMCID: PMC7462278
- DOI: 10.1093/noajnl/vdaa088
Patient-derived cells from recurrent tumors that model the evolution of IDH-mutant glioma
Abstract
Background: IDH-mutant lower-grade gliomas (LGGs) evolve under the selective pressure of therapy, but well-characterized patient-derived cells (PDCs) modeling evolutionary stages are lacking. IDH-mutant LGGs may develop therapeutic resistance associated with chemotherapy-driven hypermutation and malignant progression. The aim of this study was to establish and characterize PDCs, single-cell-derived PDCs (scPDCs), and xenografts (PDX) of IDH1-mutant recurrences representing distinct stages of tumor evolution.
Methods: We derived and validated cell cultures from IDH1-mutant recurrences of astrocytoma and oligodendroglioma. We used exome sequencing and phylogenetic reconstruction to examine the evolutionary stage represented by PDCs, scPDCs, and PDX relative to corresponding spatiotemporal tumor tissue and germline DNA. PDCs were also characterized for growth and tumor immortality phenotypes, and PDX were examined histologically.
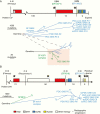
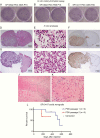
Results: The integrated astrocytoma phylogeny revealed 2 independent founder clonal expansions of hypermutated (HM) cells in tumor tissue that are faithfully represented by independent PDCs. The oligodendroglioma phylogeny showed more than 4000 temozolomide-associated mutations shared among tumor samples, PDCs, scPDCs, and PDX, suggesting a shared monoclonal origin. The PDCs from both subtypes exhibited hallmarks of tumorigenesis, retention of subtype-defining genomic features, production of 2-hydroxyglutarate, and subtype-specific telomere maintenance mechanisms that confer tumor cell immortality. The oligodendroglioma PDCs formed infiltrative intracranial tumors with characteristic histology.
Conclusions: These PDCs, scPDCs, and PDX are unique and versatile community resources that model the heterogeneous clonal origins and functions of recurrent IDH1-mutant LGGs. The integrated phylogenies advance our knowledge of the complex evolution and immense mutational load of IDH1-mutant HM glioma.
Keywords: IDH1-mutant glioma; hypermutation; intracranial xenograft; intratumoral heterogeneity and evolution; patient-derived cells.
© The Author(s) 2020. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Figures


References
-
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. - PubMed
-
- Soffietti R, Baumert BG, Bello L, et al. ; European Federation of Neurological Societies Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17(9):1124–1133. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
