Limb girdle muscular dystrophy due to LAMA2 gene mutations: new mutations expand the clinical spectrum of a still challenging diagnosis
- PMID: 32904964
- PMCID: PMC7460730
- DOI: 10.36185/2532-1900-009
Limb girdle muscular dystrophy due to LAMA2 gene mutations: new mutations expand the clinical spectrum of a still challenging diagnosis
Abstract
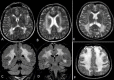
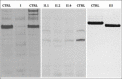
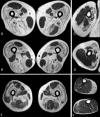
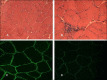
Mutations in LAMA2 gene, encoding merosin, are generally responsible of a severe congenital-onset muscular dystrophy (CMD type 1A) characterized by severe weakness, merosin absence at muscle analysis and white matter alterations at brain Magnetic Resonance Imaging (MRI). Recently, LAMA2 mutations have been acknowledged as responsible of LGMD R23, despite only few cases with slowly progressive adult-onset and partial merosin deficiency have been reported. We describe 5 independent Italian subjects presenting with progressive limb girdle muscular weakness, brain white matter abnormalities, merosin deficiency and LAMA2 gene mutations. We detected 7 different mutations, 6 of which are new. All patients showed normal psicomotor development and slowly progressive weakness with onset spanning from childhood to forties. Creatin-kinase levels were moderately elevated. One patient showed dilated cardiomyopathy. Muscle MRI allowed to evaluate the degree and pattern of muscular involvement in all patients. Brain MRI was fundamental in order to address and/or support the molecular diagnosis, showing typical widespread white matter hyperintensity in T2-weighted sequences. Interestingly these alterations were associated with central nervous system involvement in 3 patients who presented epilepsy and migraine. Muscle biopsy commonly but not necessarily revealed dystrophic features. Western-blot was usually more accurate than immunohystochemical analysis in detecting merosin deficiency. The description of these cases further enlarges the clinical spectrum of LAMA2-related disorders. Moreover, it supports the inclusion of LGMD R23 in the new classification of LGMD. The central nervous system involvement was fundamental to address the diagnosis and should be always included in the diagnostic work-up of undiagnosed LGMD.
Keywords: LAMA2 gene; brain MRI; leukoencephalopathy; limb girdle muscular dystrophy; merosin; muscle MRI.
©2020 Gaetano Conte Academy - Mediterranean Society of Myology, Naples, Italy.
Figures

Similar articles
-
LAMA2-related myopathy: Frequency among congenital and limb-girdle muscular dystrophies.Muscle Nerve. 2015 Oct;52(4):547-53. doi: 10.1002/mus.24588. Epub 2015 Aug 13. Muscle Nerve. 2015. PMID: 25663498
-
Limb girdle muscular dystrophy due to LAMA2 mutations: diagnostic difficulties due to associated peripheral neuropathy.Neuromuscul Disord. 2014 Aug;24(8):677-83. doi: 10.1016/j.nmd.2014.05.008. Epub 2014 Jun 2. Neuromuscul Disord. 2014. PMID: 24957499
-
Clinical and molecular genetic analysis of a family with late-onset LAMA2-related muscular dystrophy.Brain Dev. 2016 Feb;38(2):242-9. doi: 10.1016/j.braindev.2015.08.005. Epub 2015 Aug 21. Brain Dev. 2016. PMID: 26304763
-
LAMA2-Related Dystrophies: Clinical Phenotypes, Disease Biomarkers, and Clinical Trial Readiness.Front Mol Neurosci. 2020 Aug 5;13:123. doi: 10.3389/fnmol.2020.00123. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32848593 Free PMC article. Review.
-
The expanding phenotype of laminin alpha2 chain (merosin) abnormalities: case series and review.J Med Genet. 2001 Oct;38(10):649-57. doi: 10.1136/jmg.38.10.649. J Med Genet. 2001. PMID: 11584042 Free PMC article. Review.
Cited by
-
A Multicenter Cross-Sectional Study of the Swiss Cohort of LAMA2-Related Muscular Dystrophy.J Neuromuscul Dis. 2024;11(5):1021-1033. doi: 10.3233/JND-240023. J Neuromuscul Dis. 2024. PMID: 39213089 Free PMC article.
-
Unique genotype-phenotype correlations within LAMA2-related limb girdle muscular dystrophy in Chinese patients.Front Neurol. 2023 May 3;14:1158094. doi: 10.3389/fneur.2023.1158094. eCollection 2023. Front Neurol. 2023. PMID: 37206914 Free PMC article.
-
LAMA2-Related Muscular Dystrophy Across the Life Span: A Cross-sectional Study.Neurol Genet. 2023 Jul 19;9(5):e200089. doi: 10.1212/NXG.0000000000200089. eCollection 2023 Oct. Neurol Genet. 2023. PMID: 37476021 Free PMC article.
-
Case report: Adult-onset limb girdle muscular dystrophy in sibling pair due to novel homozygous LAMA2 missense variant.Front Neurol. 2023 Jan 27;14:1055639. doi: 10.3389/fneur.2023.1055639. eCollection 2023. Front Neurol. 2023. PMID: 36779065 Free PMC article.
-
Phenotype-genotype spectrum of a cohort of congenital muscular dystrophies: a single-centre experience from India.Neurogenetics. 2024 Oct;25(4):435-469. doi: 10.1007/s10048-024-00776-6. Epub 2024 Aug 5. Neurogenetics. 2024. PMID: 39103709
References
-
- Hohenester E, Yurchenco PD. Laminins in basement membrane assembly. Cell Adh Migr 2013;7:56-63. https://doi.org/10.4161/cam.21831 10.4161/cam.21831 - DOI - PMC - PubMed
-
- Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 2011;3 https://doi.org/10.1101/cshperspect.a004911 10.1101/cshperspect.a004911 - DOI - PMC - PubMed
-
- de los A Beytía M, Dekomien G, Hoffjan S, et al. High creatine kinase levels and white matter changes: clinical and genetic spectrum of congenital muscular dystrophies with laminin alpha-2 deficiency. Mol Cell Probes 2014;28:118-22. https://doi.org/10.1016/j.mcp.2013.11.002 10.1016/j.mcp.2013.11.002 - DOI - PubMed
-
- Tomé FM, Evangelista T, Leclerc A, et al. Congenital muscular dystrophy with merosin deficiency. C R Acad Sci III, Sci Vie 1994;317:351-7. - PubMed
-
- Bushby K, Anderson LV, Pollitt C, et al. Abnormal merosin in adults. A new form of late onset muscular dystrophy not linked to chromosome 6q2. Brain 1998;121:581-8. https://doi.org/10.1093/brain/121.4.581 10.1093/brain/121.4.581 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
