DNMT1-mediated Foxp3 gene promoter hypermethylation involved in immune dysfunction caused by arsenic in human lymphocytes
- PMID: 32905139
- PMCID: PMC7467266
- DOI: 10.1093/toxres/tfaa056
DNMT1-mediated Foxp3 gene promoter hypermethylation involved in immune dysfunction caused by arsenic in human lymphocytes
Abstract
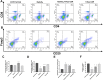
Growing evidence indicates that arsenic can cause long-lasting and irreversible damage to the function of the human immune system. It is known that forkhead box protein 3(Foxp3), which is specifically expressed in regulatory T cells (Tregs), plays a decisive role in immunoregulation and is regulated by DNA methylation. While evidence suggests that epigenetic regulated Foxp3 is involved in the immune disorders caused by arsenic exposure, the specific mechanism remains unclear. In this study, after primary human lymphocytes were treated with different doses of NaAsO2, our results showed that arsenic induced the high expression of DNMT1 and Foxp3 gene promoter methylation level, thereby inhibiting the expression levels of Foxp3, followed by decreasing Tregs and reducing related anti-inflammatory cytokines, such as interleukin 10 (IL-10) and interleukin 10 (IL-35), and increasing the ratio of CD4+/CD8+ T cells in lymphocytes. Treatment with DNA methyltransferase inhibitor 5-Aza-CdR can notably inhibit the expression of DNMT1, effectively restoring the hypermethylation of the Foxp3 promoter region in primary human lymphocytes and upregulating the expression levels of Foxp3, balancing the ratio of CD4+/CD8+ T cells in lymphocytes. It also activates the secretion of anti-inflammatory cytokines and restores the immune regulatory functions of Tregs. In conclusion, our study provides limited evidence that DNMT1-mediated Foxp3 gene promoter hypermethylation is involved in immune dysfunction caused by arsenic in primary human lymphocytes. The study can provide a scientific basis for further understanding the arsenic-induced immune dysfunction in primary human lymphocytes.
Keywords: 5-Aza-CdR; DNA methylation; DNMT1; Foxp3; arsenic; lymphocytes.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Souza ACM, Almeida MG, Pestana IA et al. . Arsenic exposure and effects in humans: a mini-review in Brazil. Arch Environ Contam Toxicol 2019;76:357–65. - PubMed
-
- Sinha D, Prasad P. Health effects inflicted by chronic low-level arsenic contamination in groundwater: a global public health challenge. J Appl Toxicol 2020;40:87–131. - PubMed
-
- Jamal Z, Das J, Ghosh S et al. . Arsenic-induced immunomodulatory effects disorient the survival-death interface by stabilizing the Hsp90/Beclin1 interaction. Chemosphere 2019, 238:124647. - PubMed
-
- Yu S, Liao WT, Lee CH et al. . Immunological dysfunction in chronic arsenic exposure: from subclinical condition to skin cancer. J Dermatol 2018;45:1271–7. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

