Alogliptin: a novel approach against cyclophosphamide-induced hepatic injury via modulating SIRT1/FoxO1 pathway
- PMID: 32905193
- PMCID: PMC7467234
- DOI: 10.1093/toxres/tfaa059
Alogliptin: a novel approach against cyclophosphamide-induced hepatic injury via modulating SIRT1/FoxO1 pathway
Abstract
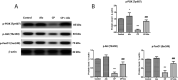
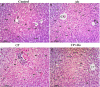
Cyclophosphamide (CP) is one of the most potent alkylating agents and is widely used in the treatment of numerous neoplastic conditions, autoimmune diseases and following organ transplantation. Due to its ability to induce oxidative stress and subsequent apoptosis, CP is affiliated with many adverse effects with special emphasis on the highly prevalent hepatotoxicity. Dipeptidyl peptidase 4 (DDP-IV) inhibitors are being rediscovered for new biological effects due to their ability to target multiple pathways, among which is the phosphoinositide 3-kinase (PI3K) and protein kinase B (Akt) axis. This could offer protection to multiple organs against reactive oxygen species (ROS) through modulating sirtuin 1 (SIRT1) expression and, in turn, inactivation of forkhead box transcription factor of the O class 1 (FoxO1), thus inhibiting apoptosis. Accordingly, the current study aimed to investigate the potential therapeutic benefit of alogliptin (Alo), a DPP-IV inhibitor, against CP-induced hepatotoxicity through enhancing PI3K/Akt/SIRT1 pathway. Forty male Wistar rats were randomly divided into four groups. The CP-treated group received a single dose of CP (200 mg/kg; i.p.). The Alo-treated group received Alo (20 mg/kg; p.o.) for 7 days with single CP injection on Day 2. Alo successfully reduced hepatic injury as witnessed through decreased liver function enzymes, increased phospho (p)-PI3K, p-Akt, superoxide dismutase (SOD) levels, SIRT1 expression, p-FoxO1 and anti-apoptotic B-cell lymphoma 2 (Bcl-2). This resulted in decreased apoptosis, as witnessed through decreased caspase-3 levels and improved histopathological picture. In conclusion, the current study succeeded to elaborate, for the first time, the promising impact of Alo in ameliorating chemotherapy-induced liver injury.
Keywords: FoxO1; SIRT1; alogliptin; cyclophosphamide; hepatotoxicity.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Bernstein JA, Garramone SM, Lower EG. Successful treatment of autoimmune chronic idiopathic urticaria with intravenous cyclophosphamide. Ann Allergy Asthma Immunol 2002;89:212–4. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

