Soy Protein-Based Hydrogel under Microwave-Induced Grafting of Acrylic Acid and 4-(4-Hydroxyphenyl)butanoic Acid: A Potential Vehicle for Controlled Drug Delivery in Oral Cavity Bacterial Infections
- PMID: 32905438
- PMCID: PMC7469417
- DOI: 10.1021/acsomega.0c02287
Soy Protein-Based Hydrogel under Microwave-Induced Grafting of Acrylic Acid and 4-(4-Hydroxyphenyl)butanoic Acid: A Potential Vehicle for Controlled Drug Delivery in Oral Cavity Bacterial Infections
Abstract
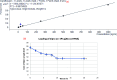
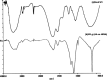
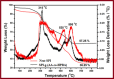
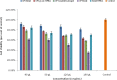
The objective of this work was to evaluate grafted soy protein isolate (SPI) for pharmaceutical applications. The present work reports the microwave-assisted preparation of soy protein isolate\grafted[acrylic acid-co-4-(4-hydroxyphenyl)butanoic acid] [SPI-g-(AA-co-HPBA)] hydrogel via graft copolymerization using N,N-methylene-bis-acrylamide and potassium persulphate as the cross-linker and initiator, respectively. The chemical and physical properties of the synthesized polymeric hydrogels were analyzed by Fourier transform infrared spectroscopy, liquid chromatography-mass spectrometry (LCMS), nuclear magnetic resonance 1H-NMR, X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA). The SEM, TEM, and XRD analyses have confirmed the formation of hydrogel SPI-g-(AA-co-HPBA) with the network structure having a layered and crystalline surface. The SPI-g-(AA-co-HPBA) hydrogel was investigated for the sustained and controlled drug delivery system for the release of model drug ciprofloxacin at basic pH for its utilization against bacterial infection in oral cavity. The drug release profile for SPI-g-(AA-co-HPBA) hydrogels was studied using LCMS at the ppb level at pH = 7.4. The synthesized hydrogel was found to be noncytotoxic, polycrystalline in nature with a network structure having good porosity, increased thermal stability, and pH-responsive behavior. The hydrogel has potential to be used as the vehicle for controlled drug delivery in oral cavity bacterial infections.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Peh K. K.; Wong C. F. Polymeric Films as Vehicle for Buccal Delivery: Swelling, Mechanical, and Bioadhesive Properties. J. Pharm. Pharm. Sci. 1999, 2, 53–61. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

