AMXI-5001, a novel dual parp1/2 and microtubule polymerization inhibitor for the treatment of human cancers
- PMID: 32905466
- PMCID: PMC7471353
AMXI-5001, a novel dual parp1/2 and microtubule polymerization inhibitor for the treatment of human cancers
Abstract
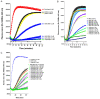
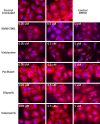
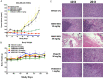
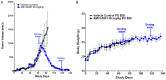
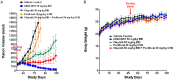
Poly (ADP-ribose) polymerase (PARP) has recently emerged as a central mediator in cancer resistance against numerous anticancer agents to include chemotherapeutic agents such as microtubule targeting agents and DNA damaging agents. Here, we describe AMXI-5001, a novel, highly potent dual PARP1/2 and microtubule polymerization inhibitor with favorable metabolic stability, oral bioavailability, and pharmacokinetic properties. The potency and selectivity of AMXI-5001 were determined by biochemical assays. Anticancer activity either as a single-agent or in combination with other antitumor agents was evaluated in vitro. In vivo antitumor activity as a single-agent was assessed in a triple-negative breast cancer (TNBC) model. AMXI-5001 demonstrates comparable IC50 inhibition against PARP and microtubule polymerization as clinical PARP inhibitors (Olaparib, Rucaparib, Niraparib, and Talazoparib) and the potent polymerization inhibitor (Vinblastine), respectively. In vitro, AMXI-5001 exhibited selective antitumor cytotoxicity across a wide variety of human cancer cells with much lower IC50s than existing clinical PARP1/2 inhibitors. AMXI-5001 is highly active in both BRCA mutated and wild type cancers. AMXI-5001 is orally bioavailable. AMXI-5001 elicited a remarkable In vivo preclinical anti-tumor activity in a BRCA mutated TNBC model. Oral administration of AMXI-5001 induced complete regression of established tumors, including exceedingly large tumors. AMXI-5001 resulted in superior anti-tumor effects compared to either single agent (PARP or microtubule) inhibitor or combination with both agents. AMXI-5001 will enter clinical trial testing soon and represents a promising, novel first in class dual PARP1/2 and microtubule polymerization inhibitor that delivers continuous and synchronous one-two punch cancer therapy with one molecule.
Keywords: AMXI-5001; BRCA; PARP inhibitor; breast cancer; cancer therapy; homologous recombination; malignancy; microtubule inhibitor; synthetic lethality.
AJCR Copyright © 2020.
Conflict of interest statement
AMXI-5001 is currently developed by AtlasMedx, Inc. Lemjabbar-Alaoui Hassan, Csaba Peto, and David Jablons may benefit financially from the development of AMXI-5001 through patents held jointly with University of California, San Francisco and AtlasMedx, Inc.
Figures

References
-
- Society AAC: American Cancer Society. Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society; 2020. volumes.
-
- O’Neil NJ, Bailey ML, Hieter P. Synthetic lethality and cancer. Nat Rev Genet. 2017;18:613–623. - PubMed
-
- Canaani D. Application of the concept synthetic lethality toward anticancer therapy: a promise fulfilled? Cancer Lett. 2014;352:59–65. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
