Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Reactions
- PMID: 32905517
- PMCID: PMC7470226
- DOI: 10.1021/acscatal.0c01842
Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Reactions
Abstract
Nickel-catalyzed reductive cross-coupling reactions have emerged as powerful methods to join two electrophiles. These reactions have proven particularly useful for the coupling of sec-alkyl electrophiles to form stereogenic centers; however, the development of enantioselective variants remains challenging. In this Perspective, we summarize the progress that has been made toward Ni-catalyzed enantioselective reductive cross-coupling reactions.
Figures
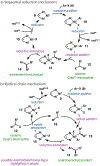
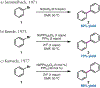
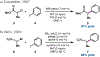

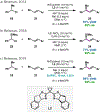
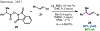
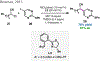
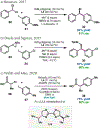

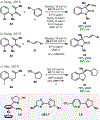
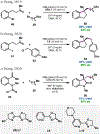
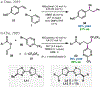
References
-
- Rudolph A; Lautens M Secondary Alkyl Halides in Transition-Metal-Catalyzed Cross-Coupling Reactions. Angew. Chem. Int. Ed 2009, 48 (15), 2656–2670. - PubMed
- Jana R; Pathak TP; Sigman MS Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-Organometallics as Reaction Partners. Chem. Rev 2011, HI (3), 1417–1492. - PMC - PubMed
-
- For selected reviews of reductive cross-couplings, see: Knappke CEI; Grupe S; Gartner D; Corpet M; Gosmini C; Jacobi von Wangelin A Reductive Cross-Coupling Reactions between Two Electrophiles. Chem. Eur. J 2014, 20 (23), 6828–6842. - PubMed
- Weix DJ Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res 2015, 48 (6), 1767–1775. - PMC - PubMed
- Gu J; Wang X; Xue W; Gong H Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Other Electrophiles: Concept and Mechanistic Considerations. Org. Chem. Front 2015, 2, 1411–1421.
- Wang X; Dai Y; Gong H Nickel-Catalyzed Reductive Couplings. Top. Curr. Chem 2016, 374 (4), 43. - PubMed
- Lucas EL; Jarvo ER Stereospecific and Stereoconvergent Cross-Couplings between Alkyl Electrophiles. Nat. Rev. Chem 2017, 1 (9), 0065.
- Richmond E; Moran J Recent Advances in Nickel Catalysis Enabled by Stoichiometric Metallic Reducing Agents. Synthesis 2018, 50 (3), 499–513.
- Goldfogel MJ; Huang L; Weix DJ Cross-Electrophile Coupling: Principles and New Reactions In Nickel Catalysis in Organic Synthesis; Ogoshi S, Ed.; Wiley, 2020; pp 183–222.
-
- For selected examples of Ni-catalyzed intramolecular RCCs, see: Yan C-S; Peng Y; Xu X-B; Wang Y-W Nickel-Mediated Inter- and Intramolecular Reductive Cross-Coupling of Unactivated Alkyl Bromides and Aryl Iodides at Room Temperature. Chem. Eur. J 2012, 18 (19), 6039–6048. - PubMed
- Xue W; Xu H; Liang Z; Qian Q; Gong H Nickel-Catalyzed Reductive Cyclization of Alkyl Dihalides. Org. Lett 2014, 16 (19), 4984–4987. - PubMed
- Konev MO; Hanna LE; Jarvo ER Intra- and Intermolecular Nickel-Catalyzed Reductive Cross-Electrophile Coupling Reactions of Benzylic Esters with Aryl Halides. Angew. Chem. Int. Ed 2016, 55 (23), 6730–6733. - PubMed
- Lucas EL; Hewitt KA; Chen P-P; Castro AJ; Hong X; Jarvo ER Engaging Sulfonamides: Intramolecular Cross-Electrophile Coupling Reaction of Sulfonamides with Alkyl Chlorides. J. Org. Chem 2020, 85 (4), 1775–1793. - PubMed
- Sanford AB; Thane TA; McGinnis TM; Chen P-P; Hong X; Jarvo ER Nickel-Catalyzed Alkyl–Alkyl Cross-Electrophile Coupling Reaction of 1,3-Dimesylates for the Synthesis of Alkylcyclopropanes. J. Am. Chem. Soc 2020, 142 (11), 5017–5023. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
