NAT1 promotes osteolytic metastasis in luminal breast cancer by regulating the bone metastatic niche via NF-κB/IL-1B signaling pathway
- PMID: 32905535
- PMCID: PMC7471372
NAT1 promotes osteolytic metastasis in luminal breast cancer by regulating the bone metastatic niche via NF-κB/IL-1B signaling pathway
Abstract
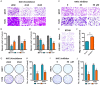
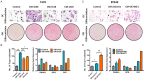
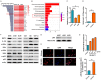
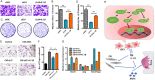
Breast cancer is a molecularly heterogeneous disease that can be subdivided into different subtypes. Compared with the other subtypes, luminal breast cancer (LBC) is considered more susceptible to bone metastasis. However, the intrinsic mechanisms remain elusive. Bioinformatics analysis of the preset study showed that N-acetyltransferase 1 (NAT1) was specifically expressed in LBC and closely correlated with bone metastasis. In addition, NAT1 could promote LBC cell migration and clonal formation, induce osteoclast differentiation and raise the Rankl/Opg ratio in osteoblasts. Our in vivo experiment demonstrated that NAT1 promoted LBC bone metastasis and bone destruction, which could be reversed by NAT1 inhibitor treatment. The result of cytokine array showed that NAT1 could significantly over activate the NF-κB signaling pathway and up-regulate the expression of IL-1B, which further worked as downstream factors in these processes. All these results demonstrated NAT1 was up-regulated in LBC and promoted the formation of bone metastatic niche and osteolytic bone metastasis through the NAT1/NF-κB/IL-1B axis. This finding may provide a new pathway to help understand the mechanisms of LBC bone metastasis and suggest a novel therapeutic and diagnostic target for its treatment.
Keywords: IL-1B; Luminal breast cancer; N-acetyltransferase 1 (NAT1); bone metastasis; bone microenvironment.
AJCR Copyright © 2020.
Conflict of interest statement
None.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China. 2015. CA Cancer J Clin. 2016;66:115–132. - PubMed
-
- Warner E. Clinical practice. Breast-cancer screening. N Engl J Med. 2011;365:1025–1032. - PubMed
LinkOut - more resources
Full Text Sources
